Abstract
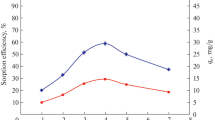
The adsorption studies of Eu(III) was investigated on 2-thenoyltrifluoroacetone (HTTA) loaded PUR foam. The adsorption conditions were optimized with respect to pH, shaking time, loading capacity and adsorbent weight. The adsorption data followed the classical Freundlich and Langmuir type isotherms successfully. The Freundlich constant (1/n) is estimated to be 0.35±0.02, reflects a surface heterogeneity of the PUR foam. Langmuir isotherm gives a saturated capacity of 0.082±0.002 mmol.g-1 suggests a monolayer coverage of the surface. The Dubinin-Radushkevich (D-R) isotherm is applied and the sorption mean free energy (E) is calculated and found to be 13.36±0.12 kJ.mol-1 suggesting that chemisorption involving chemical bonding is responsible for the adsorption process. The thermodynamic parameters such as enthalpy (ΔH), entropy (ΔS) and Gibbs free energy (ΔG) were calculated and interpreted. The positive value of ΔH indicates that the adsorption of metal ions on HTTA-loaded PUR foam is an endothermic process. A possible explanation of this endothermicity has been given. The selectivity and sensitivity of the adsorbent was also studied. The sorption of Eu(III) is greatly affected in the presence of oxalate and fluoride. The sorptive affinity of different cations towards HTTA loaded PUR foam was also discussed.
Similar content being viewed by others
References
S. Palagyi T. Braun, in: Preconcentration Techniques for Trace ElementsZ. B. Alfassi C. M. Wai (Eds), CRC Press, Boca Raton, 1992, p. 336.
M. M. Saeed A. Rusheed, Sci. Intern., 10 (1998) 273.
M. M. Saeed A. Rusheed, Radiochim. Acta, 84 (1999) 171.
D. W. Lee M. Halmann, Anal. Chim. Acta, 113 (1980) 383.
M. S. El-Shahwi M. Almehdi, J. Chromatogr., 697 (1995) 185.
A. Anka A. Sonja, Analyst, 118 (1993) 1309.
A. G. Hamza A. B. Farag T. A. Amireh Z. E. Al-Basyouni F. M. Al-Nowariser, Anal. Sci., 6 (1990) 889.
A. Chow D. Buksak, Can. J. Chem., 53 (1975) 1373.
S. Katragadda H. D. Gesser A. Chow, Talanta, 42 (1995) 725.
S. V. Beltykova N. A. Nazarenko S. V. Tsygankova, Analyst, 120 (1995) 1693.
M. M. Saeed A. Ghaffar, J. Radioanal. Nucl. Chem., 232 (1998) 271.
M. M. Saeed A. Rusheed N. Ahmed J. TÖlgyessy, Separ. Sci. Technol., 29 (1994) 2143.
M. M. Saeed S. M. Hasany M. Ahmed, Talanta, 50 (1999) 625.
S. M. Hasany M. M. Saeed M. Ahmed, Separ. Sci. Technol., 35 (2000) 379.
M. M. Saeed A. Rusheed N. Ahmed, J. Radioanal. Nucl. Chem., 211 (1996) 283.
T. Braun J. D. Navratil A. B. Farag, Polyurethane Foam Sorbents in Separation Science, CRC Press, Boca Raton, 1985.
Linear Regression Program, STLNRG, FORTRAN Scientific Subroutine Library, Peerless Eng. Service, John Wileys Sons, New York, 1984, p. 335.
W. J. Weber Jr., Adsorption Technology: A Step by Step Approach to Process Evaluation and ApplicationF. L. Slejko (Ed.), Chemical Industries Series, Vol. 19, Marcel Dekker Inc., New York, 1985, p. 16.
H. Freundlich, Colloid and Capillary Chemistry, Methuen & Co., London, 1926, p. 397.
I. Langmuir, J. Am. Chem. Soc., 37 (1915) 1139.
M. M. Dubinin L. V. Radushkevich, Proc. Acad. Sci. USSR, Phys. Chem. Sect., 55 (1947) 331.
F. Helfferich, Ion-Exchange, McGraw Hill, New York, 1962, p. 166.
F. A. Lopez C. Perez E. Sainz M. Alonso, J. Chem. Techn. Biotechnol., 62 (1995) 200.
G. R. Choppin W. F. Strazik, Inorg. Chem., 4 (1965) 1250.
I. Grenthe, Acta Chim. Scand., 18 (1964) 293.
J. M. Thomas, J. Chem. Educ., 38 (1961) 138.
J. N. Mathur, Solvent Extr. Ion Exch., 1 (1983) 349.
G. S. Shepard D. A. Thornton, Helv. Chim. Acta, 54 (1971) 2212.
H. J. Emeleus K. W. Bagnall, MTP International Review of Science, Inorganic Chemistry, Series One, Vol. 7, Butterworth and Co., Ltd., 1972, p. 282.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Saeed, M.M. Adsorption profile and thermodynamic parameters of the preconcentration of Eu(III) on 2-thenoyltrifluoroacetone loaded polyurethane (PUR) foam. Journal of Radioanalytical and Nuclear Chemistry 256, 73–80 (2003). https://doi.org/10.1023/A:1023300109423
Issue Date:
DOI: https://doi.org/10.1023/A:1023300109423