Abstract
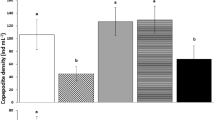
The effects of different algal foods and water temperatures on the growth and fatty acid content of the Nile Tilapia, Oreochromis niloticus L., were studied. Four types of algae, given in the same amounts as the control diet, were used as food: Microcystis aeruginosa, colonial and single-celled forms; Arthrospira fusiformis; and Scenedesmus quadricauda. The control group was fed a commercial diet of cichlid pellets, while another group was left unfed. The feeding experiment was run at 25 °C. The condition factor decreased in all algal fed fish groups, except the one fed on Microcystis colonies, whereas the control group showed no significant change. Both food quantity and quality were responsible for this result. Some short-chained fatty acids in the diets could be traced in the long-chained counter-parts in the fish tissue. Both saturated fatty acids and monounsaturated fatty acids were higher in the control vs. treatment groups, whereas the polyunsaturated fatty acids displayed no significant differences amongst any of the treatment groups studied, including the unfed group. Direct quantitative comparison of individual fatty acid in the diet vs. tissue lipids in the fish proved to be difficult due to the great capacity of these tilapias to elongate and desaturate 18 carbon acids into long-chained homologues. The effect of temperature was studied by growing the fish at 16, 20 and 25 °C. All groups were fed commercial cichlid pellets. The level of saturated and monounsaturated fatty acids increased at 20 °C, whereas polyunsaturated fatty acids showed little variation. Docosahexaenoic acid, belonging to the important ‘omega 3’ group where the first double bond starts at carbon number three, was highest at 16 °C, resulting in a markedly elevated omega-3/omega-6 ratio at that temperature.
Similar content being viewed by others
References
Admassu D. 1998. Age and Growth Determination of Tilapia, Oreochromis niloticus L. (Pisces: Cichlidae) in some Lakes on Ethiopia. School of Graduate Studies, Addis Ababa University, Addis Ababa.
Ahlgren G. 1977. Growth of Oscillatoria agardhii in chemostat culture. 1. Nitrogen and phosphorus requirements. Oikos 29: 20–224.
Ahlgren G., Gustafsson I.-B. and Boberg M. 1992. Fatty acid content and chemical composition of freshwater microalgae. J. Phycol. 28: 3–50.
Ahlgren G., Blomqvist P., Boberg M. and Gustafsson I.-B. 1994. Fatty acid content of the dorsal muscle - an indicator of fat quality in freshwater fish. J. Fish Biol. 45: 13–157.
Ahlgren G., Carlstein M. and Gustafsson I.-B. 1999. Effects of natural and commercial diets on the fatty acid quality of dorsal muscle tissue in European grayling. J. Fish Biol. 55: 114–1155, Doi: jfbi.1999.1117.
Balarin J.D. and Hatton J. 1979. Tilapia: A Guide to Their Biology and Culture in Africa. University of Sterling, Scotland, 142 pp.
Bell J.G., McVicar A.H., Park M.T. and Sargent J.R. 1991. High dietary linoleic acid affects the fatty acid compositions of individual phospholipids from tissues of Atlantic salmon (Salmo salar): association with stress susceptibility and cardiac lesion. J. Nutr. 121: 116–1172.
Boberg M., Croon L.-B., Gustafsson I.-B. and Vessby B. 1985. Platelet fatty acid composition in relation to fatty acid composition in plasma and serum lipoprotein lipids in healthy subjects with special reference to the linoleic acid pathway. Clin. Sci. 68: 58–587.
Caulton M.S. 1978. The effect of temperature and mass on routine metabolism in Sarotherodon (Tilapia) mossambica (Peters). J. Fish Biol. 13: 19–201.
Caulton M.S. 1982. Feeding metabolism and growth of tilapias: some quantitative considerations. In: Pullin R.S.V. and Lowe-McConnell R.H. (eds), The Biology and Culture of Tilapias. International Centre for Living Aquatic Resources Management (ICLARM), Proc. Vol. 7., Manila, Philippines, pp. 15–180.
Chervinski J. 1982. Environmental physiology of tilapias. In: Pullin R.S.V. and Lowe-McConnell R.H. (eds), The Biology and Culture of Tilapias. International Centre for Living Aquatic Resources Management (ICLARM), Proc. Vol. 7., Manila, Philippines, pp. 11–128.
Chorus I. and Bartram J. 1999. Toxic Cyanobacteria in Water. E & FN Spon, London.
Cohen Z. 1987. The chemicals of Spirulina. In: Vonshak A. (ed.), Spirulina Platensis (Arthrospira): Physiology, Cell Biology and Biotechnology. Taylor & Francis Ltd, London, pp. 17–204.
Cridland C.C. 1961. Laboratory experiments on the growth of Tilapia spp. The production of Tilapia esculenta under artificial conditions. Hydrobiologia 18: 17–184.
D'Abramo L.R. and Sheen S.-S. 1993. Polyunsaturated fatty acid nutrition in juvenile freshwater prawn Macrobrachium rosenbergii. AQUA 50084: 6–68.
Dempster P., Baird D.J. and Beveridge M.C.M. 1995. Can fish survive by filter-feeding on microparticles? Energy balance in tilapia grazing on algal suspensions. J. Fish Biol. 47: –17.
De Silva S.S., Gunasekera R.M. and Austin C.M. 1997. Change in the fatty acid profiles of hybrid red tilapia, Oreochromis mossambicus × O. niloticus, subjected to short-term starvation, and a comparison with changes in seawater raised fish. Aquaculture 153: 27–290.
El-Sayed M.M., Ezzat A.A., Kandeel K.M. and Shaban F.A. 1984. Biochemical studies on the lipid content of Tilapia nilotica and Sparus auratus. Comp. Biochem. Physiol. 79B: 58–594.
Einig R.G. and Ackman R.G. 1987. Omega-3 PUFA in marine oil products. JAOCS 64: 49–502.
Farkas T. 1984. Adaptation of fatty acid composition to temperature - a study on carp (Cyprinus carpio L.) liver slices. Comp. Biochem. Physiol. 79B: 53–535.
Farkas T., Kariko K. and Scengeri K. 1981. Incorporation of (1-14C) acetate into fatty acids of the crustaceans Daphnia magna and Cyclops strenus in relation to temperature. Lipids 16: 41–422.
Fowler J. and Cohen L. 1996. Practical Statistics for Field Biology. John Wiley & Sons, Chichester, 227 pp.
George E.A. 1976. Culture Centre of Algae and Protozoa. List of Strains 1976. 3rd edn. Natural Environment Resource Council, Cambridge, pp. –120.
Getachew T. 1993. The composition and nutritional status of the diet of Oreochromis niloticus in Lake Chamo, Ethiopia. J. Fish Biol. 42: 86–874.
Hazel J.R. 1984. Effects of temperature on the structure and metabolism of cell membranes in fish. Am. Physiol.: R46–470.
Henderson R.J. and Tocher D.R. 1987. The lipid composition and biochemistry of freshwater fish. Prog. Lipid Res. 26: 28–347.
James C.M., Al-Hinty S. and Salman A.E. 1989. Growth and ω3 fatty acids and amino acid composition of microalgae under different temperature regimes. Aquaculture 77: 33–351.
Kebede E. and Ahlgren G. 1996. Optimum growth conditions and light utilization efficiency of Spirulina platensis (= Arthrospira fusiformis) (Cyanophyta) from Lake Chitu, Ethiopia. Hydrobiologia 332: 9–109.
Kenyon C.N. 1972. Fatty acid composition of unicellular strains of blue-green algae. J. Bact. 109: 82–834.
Komarek J. and Lund J.W.G. 1990. What is ‘Spirulina platensis’ in fact? Algolog Studies 58: –13, Arch Hydrobiol/Supp l 85.
Lobel P.S. 1981. Trophic biology of herbivorous reef fishes: alimentary pH and digestive capacities. J. Fish Biol. 19: 20–209.
Lundstedt L. and Brett M.T. 1991. Differential growth rates of three cladoceran species in response to mono-and mixed-algal diets. Limnol. Oceonogr. 36: 15–165.
McDonald M.E. 1985. Carbon budgets for a phytoplanktivorous fish fed three different unialgal populations. Oecologia 66: 24–249.
Northcott M.E., Beveridge M.C.M. and Ross L.G. 1991. A laboratory investigation of the filteration and ingestion rates of tilapia, Oreochromis niloticus, feeding on two species of bluegreen algae. Environ. Biol. Fish 31: 7–85.
Olsen R.E., Henderson R.J. and McAndrew B.J. 1990. The conversion of linoleic acid and linolenic acid to longer chain polyunsaturated fatty acids by Tilapia (Oreochromis niloticus) in vivo. Fish Physiol. Biochem. 8: 26–270.
Piorreck M.K., Baasch H. and Pohl P. 1984. Biomass production, total protein, chlorophylls, lipids and fatty acids of freshwater green and blue-green algae under different nitrogen regims. Phytochemistry 23: 20–216.
Sargent J.R., Bell J.G., Bell M.V., Henderson R.J. and Tocher D.R. 1995. Requirement criteria for essential fatty acid. J. Appl. Ichthyol. 11: 18–198.
Staub R. 1961. Ernährungsphysiologisch-autökologische Untersuchungen an der planktischen Blaualge Oscillatoria rubescens D. C. Schweiz Z. Hydrol. 23: 8–198.
Steffens W. 1989. Principles of Fish Nutrition. Ellis Horwood, New York, pp. –384.
Tudorancea C., Fenando C.H. and Paggi J.C. 1988. Food and feeding ecology of Oreochromis niloticus (Linnaeus) juveniles in Lake Awassa (Ethiopia). Arch. Hydrobiol. 79, Suppl: 26–289.
Watanabe T., Ohta M., Kitajima C. and Fujitas S. 1982. Improvement of dietary value of brine shrimp Artemia salina for fish larvae by feeding them with ω3 highly unsaturated fatty acids. Bull Jap. Soc. Sci. Fish 48: 177–1782.
Weatherley A.H. and Gill H.S. 1987. The Biology of Fish Growth. Academic Press, London.
Zenebe T. 1997. Breeding season, fecundity, length-weight relationship and condition factor of Oreochromis niloticus L. (Pisces: Cichlidae) in Lake Tana, Ethiopia. SINET 20: 3–47.
Zenebe T., Ahlgren G., Gustafsson I.-B. and Boberg M. 1998a. Fatty acid and lipid content of Oreochromis niloticus L. in Ethiopian lakes - dietary effects of phytoplankton. Ecol. Freshw. Fish 7: 14–158.
Zenebe T., Ahlgren G. and Boberg M. 1998b. Fatty acid content of some freshwater fish of commercial importance from topical lakes in the Ethiopian Rift Valley. J. Fish Biol. 53: 98–1005.
Zenebe T. 1998c. Food and Feeding Ecology of Tilapia, Oreochromis Niloticus L. and Effects of Diet on the Lipid Quality of Fish in some Lakes in Ethiopia. School of Graduate Studies, Addis Ababa University, Addis Ababa.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tadesse, Z., Boberg, M., Sonesten, L. et al. Effects of algal diets and temperature on the growth and fatty acid content of the cichlid fish Oreochromis niloticus L. – A laboratory study. Aquatic Ecology 37, 169–182 (2003). https://doi.org/10.1023/A:1023942711822
Issue Date:
DOI: https://doi.org/10.1023/A:1023942711822