Abstract
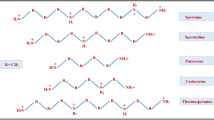
Root development is under the control of hormonal, metabolic, and environmental cues that can act on genetically-controlled developmental programmes and thus affect the plasticity of root architecture. These processes involve not only the five `classical' plant hormones, but also other growth regulators, such as polyamines. The present review emphasises the importance of polyamines in the different aspects of root development: primary root growth and lateral and adventitious root formation. Free (agmatine, putrescine, spermidine, spermine), conjugated (such as hydroxycinnamate conjugates) and macromolecule-bound polyamines are reported to be present in root systems. Modifications of their endogenous levels by inhibitor treatment, by mutation, by gene manipulation, or by exogenous treatment can have drastic effects on root development and subsequent architecture. These effects may be related to the involvement of polyamines in the control of cell division and differentiation, which plays an important role in the root apex and during lateral and adventitious root formation. The exact mechanisms of action remain to be elucidated, but accumulating evidence in plant and animal cells supports the idea that, besides biophysical effects on membranes and nucleic acids, polyamines interact with protein kinases and transcription factors and are thus involved in signal transduction pathways. The high flexibility of polyamine metabolism in response to environmental stress and the metabolic link between polyamine and ethylene synthesis strongly suggest that polyamines may play a role in environmentally-induced plasticity of root development. Moreover, polyamines may be implicated in the establishment of biotic interactions between roots and rhizospheric micro-organisms.
Similar content being viewed by others
References
Alabadi D, Aguero MS, Perez-Amador MA &; Carbonell J (1996) Arginase, arginine decarboxylase, ornithine decarboxylase, and polyamines in tomato ovaries. Plant Physiol. 112: 1237–1244.
Ali RM (2000) Role of putrescine in salt tolerance of Atropa belladonna plant. Plant Sci. 152: 173–179
Altabella T, Angel E, Biondi S, Palazón J, Bagni N &; Piñol MT (1995) Effect of the rol genes from Agrobacterium rhizogenes on polyamine metabolism in tobacco roots. Physiol. Plant. 95: 479–487
Altamura MM, Torrigiani P, Capitani F, Scaramagli S &; Bagni N (1991) De novo root formation in tobacco thin layers affected by inhibition of polyamine biosynthesis. J. Exp. Bot. 42: 1575–1582
Altamura MM, Capitani F, Cercia R, Falasca G &; Bagni N (1993) Cytological events induced by the inhibition of polyamine biosynthesis in thin layers of tobacco. Protoplasma 175: 9–16
Altman A (1989) Polyamines and plant hormones. In: Bachrach U &; Heimer YM (eds) The Physiology of Polyamines, Vol. 2 (pp. 121–145). CRC Press, Boca Raton
Aribaud M, Carré M &; Martin-Tanguy J (1995) Transglutaminaselike activity in Chrysanthemum leaf explants cultivated in vitro in relation to cell growth and hormone treatment. Plant Growth Regul. 16: 11–17
Athwal GS &; Huber SC (2002) Divalent cations and polyamines bind to loop 8 of 14-3-3 proteins, modulating their interaction with phosphorylated nitrate reductase. Plant J. 29: 119–129
Bagni N &; Pistocchi R (1991) Uptake and transport of polyamines and inhibitors of polyamine metabolism in plants. In: Slocum RD &; Flores HE (eds) Biochemistry and Physiology of Polyamines in Plants (pp. 105–118). CRC Press, Boca Raton
Bagni N, Torrigiani P &; Barbieri P (1981) Effect of various inhibitors of polyamine synthesis on the growth of Helianthus tuberosus. Med. Biol. 59: 403–409
Bell DL &; Sultan SE (1999) Dynamic phenotypic plasticity for root growth in polygonum: a comparative study. Am. J. Bot. 86: 807–819
Ben-Hayyim G, Damon JP, Martin-Tanguy J &; Tepfer D (1994) Changing root system architecture through inhibition of putrescine and feruloyl putrescine accumulation. FEBS Lett. 342: 145–148
Ben-Hayyim G, Martin-Tanguy J &; Tepfer D (1996) Changing root and shoot architecture with the rolA gene from Agrobacterium rhizogenes: interactions with gibberellic acid and polyamine metabolism. Physiol. Plant. 96: 237–243
Biondi S, Mengoli M, Mott D &; Bagni N (1993) Hairy root cultures of Hyoscyamus-musticus – effect of polyamine biosynthesis inhibitors. Plant Physiol. Biochem. 31: 51–58
Bouchereau A, Aziz A, Larher F &; Martin-Tanguy J (1999) Polyamines and environmental challenges: recent development. Plant Sci. 140: 103–125
Burtin D, Martin-Tanguy J, Paynot M &; Rossin N (1989) Effects of the suicide inhibitors of arginine and ornithine decarboxylase activities on organogenesis, growth, free polyamine and hydroxycinnamoylputrescine levels in leaf explants of Nicotiana Xanthi n.c. cultivated in vitro in a medium producing callus formation. Plant Physiol. 89: 104–110
Burtin D, Martin-Tanguy J, Paynot M, Carré M &; Rossin N (1990) Polyamines, hydroxycinnamoylputrescines and root formation in leaf explants of tobacco cultivated in vitro – effects of the suicide inhibitors of putrescine synthesis. Plant Physiol. 93: 1398–1404
Celenza JL, Grisafi PL &; Fink GR (1995) A pathway for lateral root formation in Arabidopsis thaliana. Genes Dev. 9: 2131–2142
Chattopadhyay MK, Gupta S, Sengupta DN &; Ghosh B (1997) Expression of arginine decarboxylase in seedlings of indica rice (Oryza sativa L.) cultivars as affected by salinity stress. Plant Mol. Biol. 34: 477–483
Clark DG, Gubrium EK, Barrett JE, Nell TA &; Klee HJ (1999) Root formation in ethylene-insensitive plants. Plant Physiol. 121: 53–60
Datta N, Schell MB &; Roux SJ (1987) Spermine stimulation of a nuclear NII kinase from pea plumules and its role in the phosphorylation of a nuclear polypeptide. Plant Physiol. 84: 1397–1401
Doerner P, Jorgensen JE, You R, Steppuhn J &; Lamb C (1996) Control of root growth and development by cyclin expression. Nature 380: 520–523
El Amrani A, Marie L, Aïnouche A, Nicolas J &; Couée I (2002) Genome-wide distribution and potential regulatory functions of AtATE, a novel family of miniature inverted-repeat transposable elements in Arabidopsis thaliana. Mol. Genet. Genomics 267: 459–471
El Ghachtouli N, Martin-Tanguy J, Paynot M &; Gianinazzi S (1996) First report of the inhibition of arbuscular mycorrhizal infection of Pisum sativum by specific and irreversible inhibition of polyamine biosynthesis or by gibberellic acid treatment. FEBS Lett. 358: 189–192
Ermel FF, Vizoso S, Charpentier JP, Jay-Allemand C, Catesson AM &; Couée I (2000) Mechanisms of primordium formation during adventitious root development from walnut cotyledon explants. Planta 211: 563–574
Evans PT &; Malmberg RL (1989) Do polyamines have roles in plant development? Ann. Rev. Plant Physiol. PlantMol. Biol. 40: 235–269
Flores HE (1991) Changes in polyamine metabolism in response to abiotic stress. In: Slocum R &; Flores HE (eds) The Biochemistry and Physiology of Polyamines in Plants (pp. 214–225). CRC Press, Boca Raton
Flores HE &; Galston A (1982) Analysis of polyamines in higher plants by high performance liquid chromatography. Plant Physiol. 69: 701–706
Fogel R (1985) Roots as primary producers in below-ground ecosystems. In: Fitter AH, Atkinson D, Read DJ &; Usher MB (eds) Ecological Interaction in Soil: Plants, Microbes and animals, Special Publication of the British Ecological Society, Vol. 4 (pp. 23–36). Blackwell Scientific Publication, Oxford
Fowler MR, Kirby MJ, Scott NW, Slater A &; Elliott MC (1996) Polyamine metabolism and gene regulation during the transition of autonomous sugar beet cell in suspension culture from quiescence to division. Physiol. Plant. 98: 439–446
Friedman R, Altman A &; Bachrach U (1982) Polyamines and root formation in mung bean hypocotyl cuttings. I. Effect of exogenous compounds and changes in endogenous polyamines. Plant Physiol. 70: 844–848
Friedman R, Altman A &; Bachrach U (1985) Polyamines and root formation in mung bean hypocotyl cuttings. II. Incorporation of precursors into polyamines. Plant Physiol. 79: 80–83
Fujihara S, Abe H &; Yoneyama T (1995) A new polyamine 4-aminobutylcadaverine, occurrence and its biosynthesis in root nodules of adzuki bean plant Vigna angularis. J. Biol. Chem. 270: 9932–9938
Fuller DJ, Gerner EW &; Russell DH (1977) Polyamine biosynthesis and accumulation during the G1 to S phase transition. J. Cell Physiol. 93: 81–88
Galloway GL, Malmberg RL &; Price RA (1998) Phylogenetic utility of the nuclear gene arginine decarboxylase: an example from Brassicaceae. Mol. Biol. Evol. 15: 1312–1320
Galston AW &; Flores HE (1991) Polyamines and plant morphogenesis. In: Slocum R &; Flores HE (eds) The Biochemistry and Physiology of Polyamines in Plants (pp. 175–186). CRC Press, Boca Raton
Galston AW, Kaur-Sawhney R, Altabella T &; Tiburcio AF (1997) Plant polyamines in reproductive activity and response to abiotic stress. Bot. Acta 110: 197–207
Granell A &; Carbonell J (2000) In situ hybridisation of ADC and ODC in different vegetative and reproductive tissues of the tomato. Abstract S35-3. International Society of Plant Molecular Biology
Guillet G, Poupart J, Basurco J &; De Luca V (2000) Expression of tryptophan decarboxylase and tyrosine decarboxylase genes in tobacco results in altered biochemical and physiological phenotypes. Plant Physiol. 122: 933–943
Hanfrey C, Sommer S, Mayer MJ, Burtin D &; Michael AJ (2001) Arabidopsis polyamine biosynthesis: absence of ornithine decarboxylase and the mechanism of arginine decarboxylase activity. Plant J. 27: 551–560
Hanzawa Y, Takahashi T, Michael AJ, Burtin D, Long D, Pineiro M, Coupland G &; Komeda Y (2000) ACAULIS5, an Arabidopsis gene required for stem elongation, encodes a spermine synthase. EMBO J. 19: 4248–4256
Hanzawa Y, Imai A, Michael AJ, Komeda Y &; Takahashi T (2002) Characterisation of the spermidine synthase-related gene family in Arabidopsis thaliana. FEBS Lett. 527: 176–180
Hart JJ, DiTomaso JM, Linscott DL &; Kochian LV (1992) Transport interactions between paraquat and polyamines in roots of intact maize seedlings. Plant Physiol. 99: 1400–1405
Hausman JF, Kevers C &; Gaspar T (1994) Involvement of putrescine in the inductive rooting phase of poplar shoots raised in vitro. Physiol. Plant. 92: 201–206
Hennion F &; Martin-Tanguy J (2000) Amines of the subantarctic crucifer Pringlea antiscorbutica are responsive to temperature conditions. Physiol. Plant. 109: 232–243
Hummel I, Couée I, El Amrani A, Martin-Tanguy J &; Hennion F (2002) Involvement of polyamines in root development at low temperature in the subantarctic cruciferous species Pringlea antiscorbutica. J. Exp. Bot. 53: 1463–1473
Janas KM, Cvikrovà M, Palagiewicz A &; Eder J (2000) Alterations in phenylpropanoid content in soybean roots during low temperature acclimation. Plant Physiol. Biochem. 38: 587–593
Jarvis BC, Shannon PRM &; Yasmin S (1983) Involvement of polyamines with adventitious root development in stem cuttings of mung bean. Plant Cell Physiol. 24: 677–683
Kende HK &; Zeevaart JAD (1997) The five classical plant hormones. Plant Cell 9: 1197–1210
Klepper B (1990) Root growth and water uptake. In: Stewart BA &; Nielsen DR (eds) Irrigation of Agricultural Lands (pp. 284–322). Am. Soc. Agron, Madison, Wisconsin
Kuiper I, Bloemberg GV, Noreen S, Thomas-Oates JE &; Lugtenberg BJ (2001) Increased uptake of putrescine in the rhizosphere inhibits competitive root colonization by Pseudomonas fluorescens strain WCS365. Mol. Plant-Microbe Inter. 14: 1096–1104
Lee TM (1997) Polyamine regulation of growth and chilling tolerance of rice (Oryza sativa L.) roots cultured in vitro. Plant Sci. 122: 111–117
Lee TM, Lur HS &; Chu C (1997) Role of abscissic acid in chilling tolerance of rice (Oryza sativa L.) seedlings. 2. Modulation of free polyamine levels. Plant Sci. 126: 1–10
Lepri O, Bassie L, Safwat G, Thu-Hang P, Trung-Nghia P, Holtta E, Christou P &; Capell T (2001) Over-expression of a cDNA for human ornithine decarboxylase in transgenic rice plants alters the polyamine pool in a tissue-specific manner. Mol. Genet. Genomics. 266: 303–312
Locke JM, Bryce JH &; Morris PC (2000) Contrasting effects of ethylene perception and biosynthesis inhibitors on germination and seedling growth of barley (Hordeum vulgare L.). J. Exp. Bot. 51: 1843–1849
Lund ST, Smith AG &; Hackett WP (1996) Cuttings of a tobacco mutant, rac, undergo cell divisions but do not initiate adventitious roots in response to exogenous auxin. Physiol. Plant. 114: 1197–1206
Lynch J (1995) Root architecture and plant productivity. Plant Physiol. 109: 7–13
Malamy JE &; Benfey PN (1997) Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124: 33–44
Martin-Tanguy J (2001) Metabolism and function of polyamines in plants: recent development (new approaches). Plant Growth Regul. 34: 135–148
Martin-Tanguy J &; Carré M (1993) Polyamines in gravepine microcuttings cultivated in-vitro – effects of amines and inhibitors of polyamine biosynthesis on polyamine levels and microcutting growth and development. Plant Growth Regul. 13: 269–280
Martin-Tanguy J, Tepfer D, Paynot M, Burtin D, Heisler L &; Martin C (1990) Inverse relationship between polyamine levels and the degree of phenotypic alteration induced by the rootinducing, left-hand transferred DNA from Agrobacterium rhizogenes. Plant Physiol. 92: 912–918
Mathesius U, Schalman HRM, Spaink HP, Sautter C, Rofle BG &; Djordjevic MA (1998) Auxin transport inhibition precedes root nodule formation in white clover roots and is regulated by flavonoids and derivatives of chitin oligosacharides. Plant J. 14: 23–34
Nam KH, Lee SH &; Lee J (1997) Differential expression of ADC mRNA during development and upon acid stress in soybean (Glycine max.) hypocotyls. Plant Cell Physiol. 38: 1156–1166
Niemi K, Haggman H &; Sarjala T (2002) Effects of exogenous diamines on the interaction between ectomycorrhizal fungi and adventitious root formation in Scots pine in vitro. Tree Physiol. 22: 373–381
Ober D &; Hartmann T (1999) Homospermidine synthase, the first pathway-specific enzyme of pyrrolizidine alkaloid biosynthesis, evolved from deoxyhypusine synthase. Proc. Natl. Acad. Sci. USA 96: 14777–14782
O'Connell KP, Goodman RM &; Handelsman J (1996) Engineering the rhizosphere: expressing a bias. Trends Biotechnol. 14: 83–88
Palavan-Unsal N (1987) Polyamine metabolism in the roots of Phaseolus vulgaris. Interaction of inhibitors of polyamine biosynthesis with putrescine in growth and polyamine biosynthesis. Plant Cell Physiol. 58: 565–572
Perez-Amador MA, Carbonell J &; Granell A (1995) Expression of arginine decarboxylase is induced during early fruit development and in young tissues of Pisum sativum (L). Plant Mol. Biol. 28: 997–1009
Piotrowski M, Janowitz T &; Kneifel H (2003) Plant C-N hydrolases: identification of a plant N-carbamoylputrescine amidohydrolase involved in polyamine biosynthesis. J. Biol. Chem. 278: 1708–1712
Reis DJ &; Regunathan S (2000) Is agmatine a novel neurotransmitter in brain? Trends Pharmacol. Sci. 21: 187–193
Ross J &; O'Neill D (2001) New interactions between classical plant hormones. Trends Plant Sci. 6: 2–4
Rost TL &; Bryant JA (1996) Root organization and gene expression patterns. J. Exp. Bot. 47: 1613–1628
Roy M &; Ghosh B (1996) Polyamines, both common and uncommon, under heat stress in rice (Oryza sativa) callus. Physiol. Plant. 98: 196–200
Rupniak HT &; Paul D (1978) Inhibition of spermidine and spermine synthesis leads to growth arrest of rat embryo fibroblast in G1. J. Cell Physiol. 94: 161–170
Scheres B, McKhann HI &; Van Der Berg C (1996) Roots redefined: anatomical and genetic analysis of root development. Plant Physiol. 111: 959–964
Schwartz M, Altman A, Cohen Y &; Arzee T (1986) Localization of ornithine decarboxylase and changes in polyamine content in root meristems of Zea mays. Physiol. Plant. 67: 485–492
Shen HJ &; Galston AW (1985) Correlations between polyamine ratios and growth patterns in seedling roots. Plant Growth Regul. 3: 353–363
Smith TA (1980) Plant amines. In: Bell EA &; Charlwood BV (eds) Secondary Plant Products Encyclopedia of Plant Physiology (pp. 443–460). Springer Verlag, Berlin
Smith TA (1985) Polyamines. Annu. Rev. Plant Physiol. 36: 117–143
Smith TA &; Davies PJ (1985) Separation and quantification of polyamines in plant tissue by high performance liquid chromatography of their dansyl derivatives. Plant Physiol. 78: 89–91
Smith DL &; Fedoroff NV (1995) LRP1, a gene expressed in lateral and adventitious root primordia of Arabidopsis. Plant Cell 7: 735–745
Soyka S &; Heyer AG (1999) Arabidopsis knockout mutation of ADC2 gene reveals inducibility by osmotic stress. FEBS Lett. 458: 219–223
Tarenghi E, Carré M &; Martin–Tanguy J (1995) Effects of inhibitors of polyamine biosynthesis and of polyamines on strawberry microcutting growth and development. Plant Cell Tiss. Org. Cult. 42: 47–55
Tassoni A, Van Buuren M, Franceschetti M, Fornalè S &; Bagni N (2000) Polyamine content and metabolism in Arabidopsis thaliana and effect of spermidine on plant development. Plant Physiol. Biochem. 38: 383–393
Tepfer D (1984) Transformation of several species of higher plants by Agrobacterium rhizogenes: sexual transmission of the transformed genotype and phenotype. Cell 47: 959–967
Tepfer D, Damon JP, Ben-Hayyim G, Pellegrineschi A, Burtin D &; Martin-Tanguy J (1994) Control of root system architecture through chemical and genetic alterations of polyamine metabolism In: Davis TD &; Haissig BE (eds) Biology of Adventitious Root Formation (pp. 181–189). Plenum Press, New York
Theiss C, Bohley P &; Voigt J (2002) Regulation by polyamines of ornithine decarboxylase activity and cell division in the unicellular green alga Chlamydomonas reinhardtii. Plant Physiol. 128: 1470–1479
Thomas T, Gunnia UB, Yurkow EJ, Seibold JR &; Thomas TJ (1993) Inhibition of calcium signalling in murine splenocytes by polyamines: differential effects on CD4 and CD8 T-cells. Biochem J. 291: 375–381
Thu-Hang P, Bassie L, Safwat G, Trung-Nghia P, Christou P &; Capell T (2002) Expression of heterologous Sadenosylmethionine decarboxylase cDNA in plants demonstrates that changes in S-adenosyl-L-methionine decarboxylase activity determine levels of the higher polyamines spermidine and spermine. Plant Physiol. 129: 1744–1754
Tonon G, Kevers C &; Gaspar T (2001) Changes in polyamines, auxins and peroxidase activity during in vitro rooting of Fraxinus angustifolia shoots: an auxin-independent rooting model. Tree Physiol. 21: 655–663
Vartanian N, Marcotte L &; Giraudat J (1994) Drought rhizogenesis in Arabidopsis thaliana: differential response of hormonal mutants. Plant Physiol. 104: 761–767
Wang Y, Devereux W, Stewart TM &; Casero RA (1999) Cloning and characterization of polyamine-modulated factor-1, a transcriptional cofactor that regulates the transcription of the spermidine/spermine N1-acetyltransferase gene. J. Biol. Chem. 274: 22095–22101
Watson MB, Emory KK, Piatak RM &; Malmberg RL (1998) Arginine decarboxylase (polyamine synthesis) mutants of Arabidopsis thaliana exhibit altered root growth. Plant J. 13: 231–239
Zhang A, Jennings A, Barlow PW &; Forde BG (1999) Dual pathways for regulation of root branching by nitrate. Plant Biol. 96: 6529–6534
Zheleva D, Tsoner T, Sergieu I &; Karanov EW (1994) Protective effects of exogenous polyamines against atrazine in pea plants. J. Plant Growth Regul. 13: 203–211.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Couée, I., Hummel, I., Sulmon, C. et al. Involvement of polyamines in root development. Plant Cell, Tissue and Organ Culture 76, 1–10 (2004). https://doi.org/10.1023/A:1025895731017
Issue Date:
DOI: https://doi.org/10.1023/A:1025895731017