Abstract
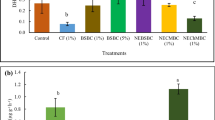
The risk of zinc (Zn) phytotoxicity in soils has increased in various regions following application of different anthropogenic materials. In order to assess the relative efficiency of Fe oxide and calcite in sorbing Zn and hence alleviating Zn phytotoxicity, we grew oilseed rape for 28 days in pots containing Zn-loaded model substrates consisting of Fe oxide (ferrihydrite)-coated sand (FOCS, 0.2–0.5 mm, 0.3 m2 ferrihydrite g−1 sand) and calcium carbonate (calcite) sand (CCS, 0.2–0.5 mm, 0.3 m2 calcite g−1 sand). Five substrates containing 5, 10, 20, 40, and 80% FOCS and supplied with ZnSO4 at a rate of 30, 100, 300, and 1000 mg Zn kg−1 were used in the cropping experiment and in an in vitro study of Zn desorption for 62 days. Plants exhibited good growth and a similar dry matter yield (DMY) at the 30 and 100 mg Zn kg−1 rates. On the other hand, DMY was markedly reduced at the 300 and, especially, at the 1000 mg Zn kg−1 rate, particularly for the substrates with the higher FOCS proportions. Symptoms of phytotoxicity (viz. chlorosis, purple colouration due to P deficiency) were apparent at such rates and were accompanied by high Zn concentrations in both shoot (average values >1000 and >1500 mg Zn kg−1 dry matter for the 300 and 1000 mg Zn kg−1 rate, respectively) and root (average values >2500 and >6000 mg Zn kg−1 dry matter for the 300 and 1000 mg Zn kg−1 rate, respectively). Total Zn uptake was maximal at 300 mg Zn kg−1. The results of water extractable Zn in the substrate after cropping and the dissolved Zn concentrations measured in substrate–water systems (desorption experiment) suggest that, on a surface area basis, calcite is more effective than Fe oxide to retain Zn and thus alleviate phytotoxicity at high Zn loadings. However, the Zn-sorption capacity of the Fe oxide cannot be neglected, particularly at low Zn loadings, where Fe oxide seems to exhibit a higher affinity for Zn – but not a higher Zn-sorption capacity – than does calcite.
Similar content being viewed by others
References
Bruemmer G, Gerth J and Tiller K G 1988 Reaction kinetics of the adsorption and desorption of nickel, zinc and cadmium by goethite. I. Adsorption and diffusion of metals. J. Soil Sci. 39, 37–52.
Brümmer G, Tiller K G, Herms U and Clayton P M 1983 Adsorption–desorption and/or precipitation–dissolution processes of zinc in soils. Geoderma 31, 337–354
Choi J M, Pak C H and Lee C W 1996 Micronutrient toxicity in French Marigold. J. Plant Nutr. 19, 901–916.
CoHort Software 1995 CoPlot Manual, CoHort Software, Minneapolis, MN. 381 p.
Diaz-Barrientos E, Madrid L, Contreras M C and Morillo E 1990 Simultaneous adsorption of zinc and phosphate on synthetic lepidocrocite. Aust. J. Soil Res. 28, 549–557.
Ebbs S D and Kochian L V 1997 Toxicity of zinc and copper to Brassica species: Implications for phytoremediation. J. Environ. Qual. 26, 776–781.
Fenter P, Geissbühler P, DiMasi E, Srajer G, Sorensen L B and Sturchio N C 2000 Surface speciation of calcite observed in situ by high-resolution X-ray reflectivity. Geochim. Cosmochim. Acta 64, 1221–1228.
Fontes R L S and Cox F R 1995 Effects of sulfur supply on soybean plants exposed to zinc toxicity. J. Plant Nutr. 18, 1893–1906.
Fontes R L S and Cox F R 1998 Zinc toxicity in soybean grown at high iron concentration in nutrient solution. J. Plant Nutr. 21, 1723–1730.
Ghanem S A and Mikkelsen D S 1988 Sorption of zinc on iron hydrous oxide. Soil Sci. 146, 15–21.
Guadalix M E and Pardo M T 1995 Zinc sorption by acid tropical soils as affected by cultivation. J. Soil Sci. 46, 47–51.
Harter R D 1991 Micronutrient adsorption–desorption reactions in soils. In Micronutrients in Agriculture. Eds. J J Mortvedt, F R Cox, L M Shuman and R M Welch. pp. 59–87. Soil Science Society of America, Madison, WI.
Kalbasi M, Racz G J and Loewen-Rudgers L A 1978 Mechanism of zinc adsorption by iron and aluminum oxides. Soil Sci. 125, 146–150.
Kiekens L 1990 Zinc. In Heavy Metals in Soils. Ed. B J Alloway, 2nd ed. Blackie Academic and Professional, Glasgow. pp. 284–305.
Lee C W, Choi J M and Pak C H 1996a Micronutrient toxicity in seed geranium (Pelargonium × hortorum Baley). J. Am. Soc. Hort. Sci. 121, 77–82.
Lee C W, Jackson M B, Duysen M E, Freeman T P and Self J R 1996b Induced micronutrient toxicity in 'Touchdown' Kentuchy Bluegrass. Crop Sci. 36, 705–712.
Lindsay W L 1991 Inorganic equilibria affecting micronutrient in soils. In Micronutrients in Agriculture. Eds. J J Mortvedt, F R Cox, L M Shuman and R M Welch. pp. 90–112. Soil Science Society of America, Madison, WI.
Lindsay W L and Norwell W A 1978 Development of a DTPA soil test for zinc, iron, manganese and copper. Soil Sci. Soc. Am. J. 42, 421–428.
Madrid L, Diaz-Barrientos E and Contreras MC 1991 Relationships between zinc and phospate adsorption on montmorillonite and an iron oxyhydroxide. Aust. J. Soil Res. 29, 239–247.
Marschner H 1986 Mineral Nutrition of Higher Plants. Academic Press, London. 674 pp.
Matar A 1992, Torrent J and Ryan J 1992 Soil and fertilizer phosphorus and crop responses in the dryland Mediterranean zone. Adv. Soil Sci. 18, 81–146.
Mortvedt J J 2000 Bioavailability of micronutrients. In Handbook of Soil Science. Ed. in chief M E Sumner. pp. D71–D86. CRC Press LLC, Boca Raton, FL.
Murphy J and Riley J P 1962. A modified single solution method for determination of phosphate in natural waters. Anal. Chim. Acta 27, 31–36.
Olson R V and Ellis R Jr 1982 Iron. In Methods of Soil Analysis, Part 2, 2nd edn. Ed. A L Page. pp. 301–312. Agronomy 9, American Society of Agronomy, Madison, WI.
Rahmatullah and Torrent J 2000 Phosphorus dynamics and uptake by wheat in a model calcite–ferrihydrite system. Soil Sci. 165, 803–812.
Randal S S and Bruce R J 1991 Zinc sorption by iron-oxide-coated sand as a function of pH. Soil Sci. Soc. Am. J. 55, 1287–1291.
Saleh M E, Saeed M K, Khalid H M I and Mostafa H E 1998 Affinity of calcium carbonate surfaces to zinc sorption. Alex. J. Agric. Res. 43, 303–315.
SAS Institute 1993 SAS/STAT User's Guide. Release 6 ed. SAS Int., Cary, NC.
Schindler P, Reinert M and Gamsjäger H 1969 Löslichkeitskonstanten und freie Bildungsenthalpien von ZnCO3 und Zn5(OH)6(CO3)2 bei 25 °. Helv. Chim. Acta 52, 2327–2332.
Shuman L M 1977 Adsorption of zinc by Fe and Al hydrous oxides as influenced by aging and pH. Soil Sci. Soc. Am. J. 40, 703–706.
Spark K M, Johnson B B and Wells J D 1995 Characterising heavymetal adsorption on oxides and oxyhydroxides. Eur. J. Soil Sci. 46, 621–631.
Sposito G 1981 The Thermodynamics of Soil Solutions. Oxford University Press, Oxford. 223 pp.
Sumner M E and Farina M P W 1986 Phosphorus interactions with other nutrients and lime in field cropping systems. Adv. Soil Sci. 5, 201–236.
Takkar P N, M S Mann, R L Bansal, N S Randhawa and H Singh 1976 Yield and uptake response of corn to zinc as influenced by phosphorus fertilization. Agron. J. 68, 942–946.
Uygur V and Rimmer D L 2000 Reactions of zinc with iron-oxide coated calcite surfaces at alkaline pH. Eur. J. Soil Sci. 51, 511–516.
Waychunas G A, Fuller C C and Davis J A 2002 Surface complexation and precipitate geometry for aqueous Zn(II) sorption on ferrihydrite I: X-ray absorption extended fine structure spectroscopy analysis. Geochim. Cosmochim. Acta 66, 1119–1137.
Weidler P 1995 Oberflächen und Porositäten syntetischer Eisenoxide. Ph.D. Thesis. Technische Universität München, Freising–Weihenstephan, Germany.
Zachara J M, Kittrick J A and Harsh J B 1988 The mechanism of Zn2+ adsorption on calcite. Geochim. Cosmochim. Acta 52, 2281–2291
Zasosky R J and Burau R G 1977 A rapid nitric–perchloric acid digestion method for multielement tissue analysis. Commun. Soil Sci. Plant Anal. 8, 425–436.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Montilla, I., Parra, M. & Torrent, J. Zinc phytotoxicity to oilseed rape grown on zinc-loaded substrates consisting of Fe oxide-coated and calcite sand. Plant and Soil 257, 227–236 (2003). https://doi.org/10.1023/A:1026289807917
Issue Date:
DOI: https://doi.org/10.1023/A:1026289807917