Abstract
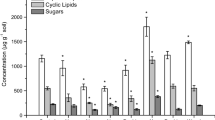
To investigate the influence of individual tree species on nitrogen (N) cycling in forests, we measured key characteristics of the N cycle in small single-species plots of five dominant tree species in the Catskill Mountains of New York State. The species studied were sugar maple (Acer saccharum), American beech (Fagus grandifolia), yellow birch (Betula alleghaniensis), eastern hemlock (Tsuga canadensis), and red oak (Quercus rubra). The five species varied markedly in N cycling characteristics. For example, hemlock plots consistently showed characteristics associated with "slow" N cycling, including low foliar and litter N, high soil C:N, low extractable N pools, low rates of potential net N mineralization and nitrification and low NO −3 amounts trapped in ion-exchange resin bags buried in the mineral soil. Sugar maple plots had the lowest soil C:N, and the highest levels of soil characteristics associated with NO −3 production and loss (nitrification, extractable NO −3 , and resin bag NO −3 ). In contrast, red oak plots had near-average net mineralization rates and soil C:N ratios, but very low values of the variables associated with NO −3 production and loss. Correlations between soil N transformations and litter concentrations of N, lignin, lignin:N ratio, or phenolic constituents were generally weak. The inverse correlation between net nitrification rate and soil C:N that has been reported in the literature was present in this data set only if red oak plots were excluded from the analysis. This study indicates that tree species can exert a strong control on N cycling in forest ecosystems that appears to be mediated through the quality of soil organic matter, but that standard measures of litter quality cannot explain the mechanism of control.
Similar content being viewed by others
References
Aber J., McDowell W., Nadelhoffer K., Magill A., Berntson G., Kamakea M. et al. 1998. Nitrogen saturation in temperate forest ecosystems. Hypotheses revisited. BioSci. 48: 921–934.
Appel H.M., Govenor H.L., D'Ascenzo M., Siska E. and Schultz J.C. 2001. Limitations of folin assays of foliar phenolics in ecological studies. J. Chem. Ecol. 27: 761–778.
Baldwin I.T., Olson R.K. and Reiners W.A. 1983. Protein binding phenolics and the inhibition of nitrification in subalpine balsam fir soils. Sol Biol. Biochem. 15: 419–423.
Bate-Smith E.C. 1977. Astringent tannins of Acer species. Phytochem. 16: 1421–1426.
Berntson G.M. and Aber J.D. 2000. Fast nitrate immobilization in N saturated temperate forest soils. Soil Biol. Biochem. 32: 151–156.
Christ M.J., Peterjohn W.T., Cumming J.R. and Adams M.B. 2002. Nitrification potentials and landscape, soil and vegetation characteristics in two Central Appalachian watersheds differing in NO3 export. For. Ecol. Man. 159: 145–158.
Dail D.B., Davidson E.A. and Chorover J. 2001. Rapid abiotic transformation of nitrate in an acid forest soil. Biogeochem. 54: 131–146.
Fahey T.J., Battles J.J. and Wilson G.F. 1998. Responses of early successional northern hardwood forests to changes in nutrient availability. Ecol. Monogr. 68: 183–212.
Ferrari J.B. 1999. Fine-scale patterns of leaf litterfall and nitrogen cycling in an old-growth forest. Can. J. For. Res.29: 291–302.
Fierer N., Schimel J.P., Cates R.G. and Zou J. 2001. Influence of balsam poplar tannin fractions on carbon and nitrogen dynamics in Alaskan taiga floodplain soils. Soil Biol. Biochem. 33: 1827–1839.
Finzi A.C. and Canham C.D. 1998. Non-additive effects of litter mixtures on net N mineralization in a southern New England forest. For. Ecol. Man. 105: 129–136.
Finzi A.C., Van Breemen N. and Canham C.D. 1998. Canopy tree-soil interactions within temperate forests: Species effects on soil carbon and nitrogen. Ecol. Appl. 8: 440–446.
Gallardo A. and Merino J. 1992. Nitrogen immobilization in leaf litter at two Mediterranean ecosystems of SW Spain. Biogeochem. 15: 213–228.
Gee G.W. and Bauder J.W. 1986. Particle size analysis. Agron. Mono. 9: 383–411, In Methods of Soil Analysis, Part 1, 2nd edition.
Goodale C.L. and Aber J.D. 2001. The long-term effects of land-use history on nitrogen cycling in northern hardwood forests. Ecol. Appl. 11: 253–267.
Gundersen P., Callensen I. and deVries W. 1998. Leaching in forest ecosystems is related to forest floor C/N ratios. Envir. Pollut. 102: 403–407.
Hagerman A.E. and Klucher K.M. 1986. Tannin-protein interactions. In: Cody V., Middleton E. and Harborne J. (eds), Plant Flavonoids in Biology and Medicine: Biochemical, Pharmacological and Structure Activity Relationships. Alan R. Liss, NY, USA, pp. 67–76.
Hartzfeld P.W., Forkner R., Hunter M.D. and Hagerman A.E. 2002. Determination of hydrolyzable tannins (gallotannins and ellagitannins) after reaction with potassium iodate. J. Agric. Chem. 50: 1285–1290.
Hattenschwiler S. and Vitousek P. 2000. The role of polyphenols in terrestrial ecosystem nutrient cycling. Trends Ecol. Evol. 15: 238–243.
Hobbie S.E. 1992. Effects of plant species on nutrient cycling. Trends Ecol. Evol. 7: 336–339.
Houston D.R. 1994. Major new tree disease epidemics: Beech bark disease. Ann. Rev. Phytopath. 32: 75–87.
Johnson D.W., Cheng W. and Burke I.C. 2000. Biotic and abiotic nitrogen retention in a variety of forested soils. Soil Sci. Soc. Am. J. 64: 1503–1514.
Knoepp J.D. and Swank W.T. 1998. Rates of nitrogen mineralization across an elevation and vegetation gradient in the southern Appalachians. Plant and Soil 204: 235–241.
Lewis G.P. and Likens G.E. 2000. Low stream nitrate concentrations associated with oak forests on the Allegheny High Plateau of Pennsylvania. Water Res. Resear. 36: 3091–3094.
Lovett G.M. and Rueth H. 1999. Soil nitrogen transformations in beech and maple stands along a nitrogen deposition gradient. Ecol. Appl. 9: 1330–1344.
Lovett G.M., Weathers K.C. and Sobczak W. 2000. Nitrogen saturation and retention in forested watersheds of the Catskill Mountains NY. Ecol. Appl. 10: 73–84.
Lovett G.M., Weathers K.C. and Arthur M.A. 2002. Control of nitrogen loss from forested watersheds by soil carbon:nitrogen ratio and tree species composition. Ecosys. 5: 712–718.
Magill A.H., Aber J.D., Bentson G.M., McDowell W.H., Nadelhoffer K.J., Melillo J.M. et al. 2000. Long-term nitrogen additions and nitrogen saturation in two temperate forests. Ecosys. 3: 238–253.
McIntosh R.P. 1962. The forest cover of the Catskill Mountain region, New York, as indicated by land survey records. Am. Mid. Nat. 68: 409–423.
McIntosh R.P. 1972. Forests of the Catskill Mountains, New York. Ecol. Monogr. 42: 143–161.
McNulty S.G., Aber J.D. and Boone R.D. 1991. Spatial changes in forest floor and foliar chemistry of spruce-fir forests across New England. Biogeochem. 14: 13–29.
McNulty S.G., Aber J.D. and Newman S.D. 1996. Nitrogen saturation in a high-elevation spruce-fir stand. For. Ecol. Manage. 84: 109–121.
Melillo J.M., Aber J.D. and Muratore J.F. 1982. Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology 63: 621–626.
Mladenoff D.J. 1987. Dynamics of nitrogen mineralization and notification in hemlock and hardwood tree fall gaps. Ecology 68: 1171–1180.
Murdoch P.S. and Stoddard J.L. 1992. The role of nitrate in the acidification of streams ion the Catskill Mountains of New York. Water Resour. Res. 28: 2707–2720.
Nadelhoffer K.J., Downs M.R. and Fry B. 1999a. Sinks for 15-N enriched additions to an oak forest and a red pine plantation. Ecol. Appl. 9: 72–86.
Nadelhoffer K.J., Emmet B., Gundersen P., Kjonaas O.J., Koopmans C.J., Schleppi P. et al. 1999b. Nitrogen deposition makes a minor contribution to carbon sequestration in temperate forests. Nature 398: 145–148.
Northup R.R., Yu Z., Dahlgren R.A. and Vogt K.A. 1995. Polyphenol control of nitrogen release from pine litter. Nature 377: 227–229.
Ollinger S.V., Smith M.L., Martin M.E., Hallett R.A., Goodale C.L. and Aber J.D. 2002. Regional variation in foliar chemistry and N cycling among forests of diverse history and composition. Ecol. 83: 339–355.
Orwig D.A. and Foster D.R. 1998. Forest response to the introduced hemlock woolly adelgid in southern New England, USA. J. Torrey Bot. Soc. 125: 60–73.
Pastor J., Aber J.D., McClaugherty C.A. and Melillo J.M. 1984. Aboveground production and N and P cycling along a nitrogen mineralization gradient on Blackhawk Island, Wisconsin. Ecology 65: 256–268.
Peterjohn W.T., Foster C.J., Christ M.J. and Adams M.B. 1999. Patterns of nitrogen availability within a forested watershed exhibiting symptoms of nitrogen saturation. For. Ecol. Manage. 119: 247–257.
Rich J.L. 1934. Glacial geology of the Catskill Mountains. NYS Museum Bull. 299: 1–180.
Robertson J.B. and Van Soest P.J. 1981. The detergent system of analysis and its application to human foods. In: James W.P.T. and Theander O. (eds), The Analysis of Dietary Fiber. Marcell Dekker, New York, NY, USA.
SAS Institute 1989. SAS/STAT User's Guide, Version 6. SAS Institute, Inc., Cary, North Carolina, USA.
Schimel J.P., Cates R.G. and Reuss R. 1998. The role of balsam poplar secondary chemicals in controlling soil nutrient dynamics through succession in the Alaskan taiga. Biogeochem. 42: 221–234.
Schimel J.P., Cleve K.V., Cates R.G., Clausen T.P. and Reichardt P.B. 1996. Effects of balsam poplar (Populus balsamifera) tannins and low molecular weight phenolics on microbial activity in taiga floodplain soil: implications for changes in N cycling during succession. Can. J. Bot. 74: 84–90.
Schultz J.C. and Baldwin I.T. 1982. Oak leaf quality declines in response to defoliation by gypsy moth larvae. Science 217: 149–151.
Scott N.A. and Binkley D. 1997. Foliage litter quality and annual net N mineralization: comparison across North American forested sites. Oecologia (Berlin) 111: 151–159.
Stevenson F.J. and Swaby R.J. 1964. Nitrosation of soil organic matter: I. Nature of gases evolved during nitrous acid treatment of lignins and humic substances. Soil Sci. Soc. Am. Pro. 28: 773–778.
Stoddard J.L. and Murdoch P.S. 1991. Catskill Mountains. In: Charles D.F. (ed.), Acidic Deposition and Aquatic Ecosystems:Regional Case Studies. Springer-Verlag, New York, pp. 237–271.
Stump L.M. and Binkley D. 1993. Relationships between litter quality and nitrogen availability in Rocky Mountain forests. Can. J. For. Res. 23: 492–502.
Swain T. and Hillis W.E. 1959. The phenolic constituents of Prunus domestica. I. The quantitative analysis of phenolic constituents. J. Sci. Food Agr. 10: 63–68.
Swain T. and Goldstein J.L. 1964. The quantitative analysis of phenolic compounds. In: Pridham J.B. (ed.), Methods in Polyphenol Chemistry. Pergamon Press, Oxford, England, pp. 131–145.
Templer P.H. 2001. Direct and indirect effects of tree species on forest nitrogen retention in the Catskill Mountains NY. PhD Dissertation, Cornell University, Ithaca, NY, USA.
Venterea R.T., Lovett G.M., Groffman P.M. and Schwarz P.A. Landscape patterns of soil nitrate production in a northern hardwood-conifer forest. Soil Sci. Soc. Am. J. (in press).
Vitousek P.M. and Reiners W.A. 1975. Ecosystem succession and nutrient retention: A hypothesis. Bio-Sci. 25: 376–356.
Vitousek P.M., Walker L.R., Whiteaker L.D., Mueller-Dombois D. and Matson P.A. 1987. Biological invasion by Myrica faya alters ecosystem development in Hawaii. Science 238: 802–804.
Zimmer M. 1999. The fate and effects of ingested hydrolyzable tannins in Porcellio scaber. Journal of Chemical Ecology 25: 611–628.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lovett, G.M., Weathers, K.C., Arthur, M.A. et al. Nitrogen cycling in a northern hardwood forest: Do species matter?. Biogeochemistry 67, 289–308 (2004). https://doi.org/10.1023/B:BIOG.0000015786.65466.f5
Issue Date:
DOI: https://doi.org/10.1023/B:BIOG.0000015786.65466.f5