Abstract
Burns, non-healing wounds and pressure sores cause extensive damage to the skin leading to infection and loss of precious body fluids. Despite advances in burn management the mortality rate continues to be high and the search for an economical and easily available dressing to control burn wound infection continues. Autologous skin has limited availability and is associated with additional scarring. Conventional dressings require frequent changes which can be painful and may even require anaesthesia.
Amnion is an excellent biological dressing and its use in the treatment of burns has special appeal in India as there are religious barriers to the acceptance of bovine and porcine skin.
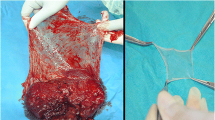
Lyophilised, irradiated amnion provided for the first time in the country by the Tata Memorial Hospital Tissue Bank was evaluated as a temporary biological dressing. It was used to treat 35 patients with burns, 21 patients with bedsores and non-healing ulcers and the skin graft donor sites of 11 patients.
The amnion was easy to handle and stuck well to the raw wound bed. An open dressing was used in most of the second degree burns which healed with hyperemia and early pigmentation. In patients with third degree burns, ulcers or skin graft donor sites, closed dressings were used. The exudate and induration were reduced and patients were more comfortable and experienced less pain. There was healthy granulation with good re-epithelialisation. Amnion was not used in patients with infected third degree burns.
Similar content being viewed by others
References
Bapat C.V. and Kothary P.M. (1974) Preliminary report on acceleration of wound healing by amnion membrane graft. Indian J. Med. Res. 62(9): 1342–1346.
Bennett J.P., Matthews R. and Faulk W.P. (1980) Treatment of chronic ulceration of the legs with human amnion. Lancet 1: 1153–1156.
Bose B. (1979) Burn wound dressing with human amniotic membrane. Ann. R. Coll. Surg. Engl. 1: 444–447.
Boyne P.J. (1968) Review of the literature on cryopreservation of bone. Cryobiology 4: 341–357.
Burgess W.H., Grieb T., Drohan W.N., Archibald L.K. and Kainer M.A. (2002) Musculoskeletal tissue allograft transplantation safety: Recent advances in sterilisation methods may prevent bacterial infections and maintain tissue function. Paper presented at the meeting of the Second Technical Advisory Committee of the IAEA Radiation and Tissue Banking Programme, Vienna, 2–4 December.
Burgos H. (1983) Angiogenic and growth factors in human amniochorion and placenta. Eur. J. Clin. Invest. 13: 289.
Burleson R. and Eiseman B. (1973) Mechanisms of antibacterial effect of biologic dressings. Ann. Surg. 177(2): 181–186.
Burwell R.G., Gowland G. and Dexter F. (1963) Studies in the transplantation of bone: VI Further observations concerning the antigenicity of homologous cortical and cancellous bone. J. Bone Joint Surg. Br. 45: 597–608.
Colocho G., Graham WP., Green AE., Metheson D.W. and Lynch D. (1974) Human amniotic membrane as a physiological wound dressing. Arch. Surg. 109: 370–373.
Conrad E.U., Gretch D.R., Obermeyer K.R., Moogk M.S., Sayers M., Wilson J.J. and Strong M. (1995) Transmission of the hepatitis-C virus by tissue transplantation. J. Bone Joint Surg. 77A(2): 214–224.
Conway B. and Tomford W.W. (1992) Radiosensitivity of human immunodeficiency virus type 1. Clin. Infect. Diseases 14: 978–979.
Conway B., Tomford W., Mankin H., Hirsch M.S. and Shooley R.T. (1991) Radiosensitivity of HIV-1. Potential application to sterilisation of bone allografts. AIDS 5: 608–609.
Davis J.W. (1910) Skin transplantation with a review of 550 cases at the Johns Hopkins Hospital. Johns Hopkins Med. J. 15: 307.
Faulk W.P., Matthews R.N., Stevens P.J., Bennett J.P., Burgos H. and Hsi B. (1980) Human amnion as an adjunct in wound healing. Lancet 1: 1156–1158.
Fideler B.M., Vangsness C.T., Moore T., Li Z. and Rasheed S. (1994) Effects of gamma irradiation on the human immunodeficiency virus. J. Bone Joint Surg. 76-A: 1032–1035.
Gruss J.S. and Jirsch D.W. (1978) Human amniotic membrane: A versatile wound dressing. Can. Med. Assoc. J. 118: 1237–1246.
Gupta M., Gupta O.K., Yaduvanshi R.K. and Upadhyaya J. (1993) Burn epidemiology: The pink city scene. Burns 19: 47–51.
IAEA (1970) Sterilisation and preservation of biological tissues by ionising radiation. International Atomic Energy Agency, Vienna.
IAEA (1973) The radiation sterilisation of medical and biological materials. Technical Report Series No. 149, International Atomic Energy Agency, Vienna.
Kumar A., Singh R., Dass P., Chauhan U.S. and Kumar P. (2000) Microbiological studies and applications of radiation sterilised human amniotic membrane. NAC 2000, March 2000, Mumbai 189–191.
Lobo Gajiwala A. (2003) Setting up a tissue bank in India: The Tata Memorial Hospital experience. Cell and Tissue Banking 4: 193–201.
Lin S.N., Lai C.S., Hou M.F. and Yang C. (1985) Amnion overlay skin autografts. Burns 11: 374–378.
Martin L.S., Mc Dougal J.S. and Loskoski S.L. (1985) Disinfection and inactivation of the human T-lymphotrophic virus type III/lymphadenopathy-associated virus. J. Infect. Dis. 152: 400–403.
Matthew R.N., Bennett J.P. and Faulk W.P. (1981) Wound healing using amniotic membranes. Br. J. Plast. Surg. 34: 76–78.
Mc Dougal J.S., Martin L.S., Cart S.P. et al. (1985) Thermal inactivation of the acquired immunodeficiency syndrome virus human T-lymphotrophic virus III/antihemophilic factor. J. Clin. Invest. 76: 875–877.
Pelkar R.R., McKay J. Jr., Troiano N. et al. (1989) Allograft incorporation: A biochemical evaluation in a rat model. J. Orthop. Res. 7: 585–589.
Prewett A., O'Leary R. and Harrell J. (1991) Kinetic evaluation of the penetration of ethanol solutions containing virucidal agents through mid-diaphyseal cortical bone. Osteotech. Tech. Rept., Shrewsbury, NJ, Osteotech Inc.
Quinby W.C., Hoover H.C., Scheflan M., Walters P.T., Slavin S.A. and Bondoc C.C. (1982) Clinical trials off amniotic membranes in burn wound care. Plast. Recon. Surg. 70(6): 711–717.
Rao T.V. and Chandrasekharam V. (1981) Use of dry human and bovine amnion as a biological dressing. Arch. Surg. 116: 891–896.
Rejzek A., Weyer F., Eichberger R. and Gebhart W. (2001) Physical changes of amniotic membranes through glycerolisation for the use as an epidermal substitute: Light and electron microscope studies. Cell Tissue Banking 2: 95–102.
Resnick L., Veren K., Salahuddin S.Z., Tondreau S. and Markham P. (1986) Stability and inactivation of HTLV-III/LAV under clinical and laboratory environments. JAMA 255 (14): 1887–1891.
Robson M.C. and Krizek T.J. (1973) The effect of human amniotic membranes on the bacterial population of infected rat burns. Ann. Surg. 177 (2): 144–149.
Robson M.C., Krizek T.J., Koss N. and Samburg H. (1973) Amniotic membrane as a temporarywound dressing. Surg. Gynecol. Obstet. 136: 904–906.
Roushdy H.M., El-Bazza Z.E., Abu-Shady M.R., Shihab A. and El-Hifnawi H.M.N. (1999) Radiation sterilisation off human amniotic membranes applicable in wound treatment and plastic surgery. Egypt J. Biomed. Sci. 4: 183–194.
Sabella N. (1913) Use of fetal membranes in skin grafting. Med. Rec. NY 83: 478.
Simonds R.J., Scott D., Holmberg, Richard L. Hurwitz, Theresa R. Coleman, Scott Bottenfield, Lois J. Conley, Sherry H. Kohlenberg, Kenneth G. Castro, Beverly A. Dahan, Charles A. Schable, Mark A. Rayfield and Martha F. Rogers (1992) Transmission of human immunodeficiency virus type 1 from a seronegative organ and tissue donor. N. Engl. J. Med. Mar. 12: 726–732.
Sinha R. (1990) Amniotic membrane in the treatment of burn injury. Indian J. Surg. January 11–17.
Spire B., Barre-Sinoussi F., Dormont D. et al. (1985) Inactivation of lymphadenopathy-associated virus by heat, gamma rays, and ultraviolet light. Lancet 1: 188–189.
Stern W. (1913) The grafting of preserved amniotic membrane to burned and ulcerated skin surfaces substituting skin grafts. JAMA 13: 973–974.
Subrahmanyam M. (1994) Honey-impregnated gauze versus amniotic membrane in the treatment of burns. Burns 20(4): 331–333.
Subrahmanyam M. (1995) Amniotic membrane as a cover or microskin grafts. Br. J. Plast. Surg. 48: 477–478.
Troensegaard-Hansen E. (1950) Amniotic grafts in chronic skin ulceration. Lancet 1: 859–860.
Unger M.G. and Roberts M. (1976) Lyophilised amniotic membrane on graft donor sites. Br. J. Plast. Surg. 29: 99–101.
Ward D.J. and Bennett J.P. (1984) The long-term results of the use of human amnion in the treatment of leg ulcerss. Br. J. Plast. Surg. 37: 191–193.
Ward D.J., Bennett J.P., Burgos H. and Fabre J. (1989) The healing of chronic venous leg ulcers with prepared human amnion. Br. J. Plast. Surg. 42: 463–467.
Yousof N. (1994) The use of gamma irradiation for sterilisation of bones and amnion. Mal. J. Nuc. Sc. 12(1): 243–251.
Yousof N. (1999) Quality system for the radiation sterilisation of tissue grafts. In: Phillips G.O., Strong D.M., von Versen R. and Nather A. (eds) Advances in Tissue Banking, Vol. 3, pp 257–281. World Scientific, Singapore.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gajiwala, K., Gajiwala, A.L. Evaluation of Lyophilised, Gamma-Irradiated Amnion as a Biological Dressing. Cell Tissue Banking 5, 73–80 (2004). https://doi.org/10.1023/B:CATB.0000034076.16744.4b
Issue Date:
DOI: https://doi.org/10.1023/B:CATB.0000034076.16744.4b