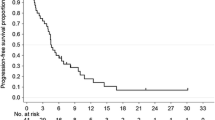
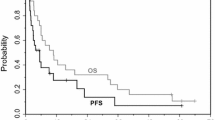
Abstract
Background: We conducted a dose escalation study combining cisplatin, irinotecan, and capecitabine (CIC), aiming to establish the maximum tolerated doses (MTD), side effect profile, and dose-limiting toxicity (DLT) of this novel regimen. Patients and methods: Intravenous cisplatin and irinotecan were to be administered on days 1 and 8, and oral capecitabine on days 1–14 of a 3-week cycle. The study was conducted in three parts. Part A: escalating doses of irinotecan (40→80 mg/m2) and capecitabine (1000→3300 mg/d) combined with a fixed dose of cisplatin (30 mg/m2). Part B: escalating doses of irinotecan (MTD-A→MTD-A+40 mg/m2) with fixed doses of cisplatin (20 mg/m2) and capecitabine (MTD-A level). Part C: escalating doses of capecitabine (1300 mg/d→2600 mg/d) with fixed doses of cisplatin (20 mg/m2) and irinotecan (60 mg/m2). Results: Of 51 eligible patients 27 (53%) were male, median age was 58 years and 88% had PS 0–1. Major primary disease sites were colorectal (24%), unknown (14%), stomach (14%), and pancreas (12%). MTD-A was cisplatin 30 mg/m2, irinotecan 60 mg/m2, capecitabine 1000 mg/d and MTD-B was cisplatin 20 mg/m2, irinotecan 90 mg/m2, capecitabine 1000 mg/d. An MTD was not formally established for part C. DLTs consisted of infection with neutropenia (1), diarrhea and fatigue (1), hypokalemia (1), diarrhea and febrile neutropenia (1) and C2 delay of ≥2 weeks or 25% dose reduction in C1 due to neutropenia or thrombocytopenia (6). Seven patients had a partial response to treatment (four colorectal, one SCLC, one NSCLC, one unknown primary), twenty seven SD (53%), twelve PD (24%) and five NE (10%). Conclusion: CIC was associated with moderate toxicity and only modest antitumor activity. We conclude that this regimen has insufficient activity to justify further study in the phase II setting.
Similar content being viewed by others
References
Dimery IW, Hong, WK: Overview of combined modality therapies for head and neck cancer. J Natl Cancer Inst 85: 95–111, 1993
Enzinger PC, Ilson DH, Kelsen DP: Chemotherapy in esophageal cancer. Semin Oncol 26: 12–20, 1999
Thomas GM: Concurrent chemotherapy and radiation for locally advanced cervical cancer: the new standard of care. Semin Radiat Oncol 10: 44–50, 2000
Masumoto N, Nakano S, Esaki T, Fujishima H, Tatsumoto T, Niho Y: Inhibition of cis-diamminedichloroplatinum (II)-induced DNA interstrand cross-link removal by 7-ethyl-10-hydroxy-camptothecin in HST-1 human squamous-carcinoma cells. Int J Cancer 62: 70–75, 1995
Ma J, Maliepaard M, Nooter K, Boersma AW, Verweij J, Stoter G, Schellens JH: Synergistic cytotoxicity of cisplatin and topotecan or SN-38 in a panel of eight solid-tumor cell lines in vitro. Cancer Chemother Pharmacol 41: 307–316, 1998
DeVore RF, Johnson DH, Crawford J, Garst J, Dimery IW, Eckardt J, Eckhardt SG, Elfring GL, Schaaf LJ, Hanover CK, Miller LL: Phase II study of irinotecan plus cisplatin in patients with advanced non-small-cell lung cancer. J Clin Oncol 17: 2710–2720, 1999
Sugiyama T, Yakushiji M, Noda K, Ikeda M, Kudoh R, Yajima A, Tomoda Y, Terashima Y, Takeuchi S, Hiura M, Saji F, Takahashi T, Umesaki N, Sato S, Hatae M, Ohashi Y: Phase II study of irinotecan and cisplatin as first-line chemotherapy in advanced or recurrent cervical cancer. Oncology 58: 31–37, 2000
Jagasia MH, Langer CJ, Johnson DH, Yunus F, Rodgers JS, Schlabach LL, Cohen AG, Shyr Y, Carbone DP, Devore RF: Weekly irinotecan and cisplatin in advanced non-small cell lung cancer: a multicenter phase II study. Clin Cancer Res 7: 68–73, 2001
Ilson DH, Saltz L, Enzinger P, Huang Y, Komblith A, Gollub M, O'Reilly E, Schwartz G, DeGroff J, Gonzalez G, Kelsen DP: Phase II trial of weekly irinotecan plus cisplatin in advanced esophageal cancer. J Clin Oncol 17: 3270–3275, 1999
Boku N, Ohtsu A, Shimada Y, Shirao K, Seki S, Saito H, Sakata Y, Hyodo I: Phase II study of a combination of irinotecan and cisplatin against metastatic gastric cancer. J Clin Oncol 17: 319–323, 1999
Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M, Gruia G, Awad L, Rougier P: Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet 355: 1041–1047, 2000
Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta N, Elfring GL, Miller LL: Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med 343: 905–914, 2000
Efficacy of intravenous continuous infusion of fluorouracil compared with bolus administration in advanced colorectal cancer. Meta-analysis Group In Cancer. J Clin Oncol 16: 301–308, 1998
Liu G, Franssen E, Fitch MI, Warner E. Patient preferences for oral versus intravenous palliative chemotherapy. J Clin Oncol 15: 110–115, 1997
Vinciguerra V, Degnan TJ, Sciortino A, O'Connell M, Moore T, Brody R, Budman D, Eng M, Carlton D: A comparative assessment of home versus hospital comprehensive treatment for advanced cancer patients. J Clin Oncol 4: 1521–1528, 1986
Miwa M, Ura M, Nishida M, Sawada N, Ishikawa T, Mori K, Shimma N, Umeda I, Ishitsuka H: Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumors by enzymes concentrated in human liver and cancer tissue. Eur J Cancer 34: 1274–1281, 1998
Van Cutsern E, Twelves C, Cassidy J, Allman D, Bajetta E, Boyer M, Bugat R, Findlay M, Frings S, Jahn M, McKendrick J, Osterwalder B, Perez-Manga G, Rosso R, Rougier P, Schmiegel WH, Seitz JF, Thompson P, Vieitez JM, Weitzel C, Harper P: Xeloda Colorectal Cancer Study Group: Oral capecitabine compared with intravenous fluorouracil plus leucovorin in patients with metastatic colorectal cancer: results of a large phase III study. J Clin Oncol 19: 4097–4106, 2001
Hoff PM, Ansari R, Batist G, Cox J, Kocha W, Kuperminc M, Maroun J, Walde D, Weaver C, Harrison E, Burger HU, Osterwalder B, Wong AO, Wong R: Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients with metastatic colorectal cancer: results of a randomized phase III study. J Clin Oncol 19: 2282–2292, 2001
Rischin D, White MA, Matthews JP, Toner GC, Watty K, Sulkowski AJ, Clarke JL, Buchanan L: A randomised cross-over trial of chemotherapy in the home: patient preferences and cost analysis. Med J Aust 173: 125–127, 2000
Blum JL, Jones SE, Buzdar AU, LoRusso PM, Kuter I, Vogel C, Osterwalder B, Burger HU, Brown CS, Griffin T: Multicenter phase II study of capecitabine in paclitaxel-refractory metastatic breast cancer. J Clin Oncol 17: 485–93, 1999
Van Cutsem E, Findlay M, Osterwalder B, Kocha W, Dalley D, Pazdur R, Cassidy J, Dirix L, Twelves C, Allman D, Seitz JF, Scholmerich J, Burger HU, Verweij J: Capecitabine, an oral fluoropyrimidine carbamate with substantial activity in advanced colorectal cancer: results of a randomized phase II study. J Clin Oncol 18: 1337–1345, 2000
Van Cutsem E, Blijham GH: Irinotecan versus infusional 5-fluorouracil: a phase III study in metastatic colorectal cancer following failure on first-line 5-fluorouracil. V302 Study Group. Semin Oncol 26: 13–20, 1999
Cunningham D, Glimelius B: A phase III study of irinotecan (CPT-11) versus best supportive care in patients with metastatic colorectal cancer who have failed 5-fluorouracil therapy. V301 Study Group. Semin Oncol 26: 6–12, 1999
de Jonge MJ, Sparreboom A, Planting AS, van der Burg ME, de Boer-Dennert MM, ter Steeg J, Jacques C, Verweij J: Phase I study of 3-week schedule of irinotecan combined with cisplatin in patients with advanced solid tumors. J Clin Oncol 18: 187–194, 2000
Masuda N, Fukuoka M, Fujita A, Kurita Y, Tsuchiya S, Nagao K, Negoro S, Nishikawa H, Katakami N, Nakagawa K, Niitani H: A phase II trial of combination of CPT-11 and cisplatin for advanced non-small-cell lung cancer. CPT-11 Lung Cancer Study Group. Br J Cancer 78: 251–256, 1998
Nakano T, Chahinian AP, Shinjo M, Togawa N, Tonomura A, Miyake M, Ninomiya K, Yamamoto T, Higashino K: Cisplatin in combination with irinotecan in the treatment of patients with malignant pleural mesothelioma: a pilot phase II clinical trial and pharmacokinetic profile. Cancer 85: 2375–2384, 1999
Cassidy J, Twelves C, Cameron D, Steward W, O'Byrne K, Jodrell D, Banken L, Goggin T, Jones D, Roos B, Bush E, Weidekamm E, Reigner B: Bioequivalence of two tablet formulations of capecitabine and exploration of age, gender, body surface area, and creatinine clearance as factors influencing systemic exposure in cancer patients. Cancer Chemother Pharmacol 44: 453–460, 1999
Ratain MJ: Dear doctor: we really are not sure what dose of capecitabine you should prescribe for your patient. J Clin Oncol 20: 1434–1435, 2002
Shirao K, Shimada Y, Kondo H, Saito D, Yamao T, Ono H, Yokoyama T, Fukuda H, Oka M, Watanabe Y, Ohtsu A, Boku N, Fujii T, Oda Y, Muro K, Yoshida S: Phase I–II study of irinotecan hydrochloride combined with cisplatin in patients with advanced gastric cancer. J Clin Oncol 15: 921–927, 1997
Saltz LB, Kanowitz J, Kemeny NE, Schaaf L, Spriggs D, Staton BA, Berkery R, Steger C, Eng M, Dietz A, Locker P, Kelsen DP: Phase I clinical and pharmacokinetic study of irinotecan, fluorouracil, and leucovorin in patients with advanced solid tumors. J Clin Oncol 14: 2959–2967, 1996
Ilson D, O'Reilly E, Sharma S, Saltz L, Maki RG, Schrag D, Joseph J, Kelsen D: A phase I trial of cisplatin, fluorouracil, and escalating doses of weekly irinotecan in patients with solid tumor malignancies. Proc Am Soc Clin Oncol, 2002 (Abstract #566)
Schleucher N, Tewes M, Achterrath W, Boiko P, Eberhardt W, Wilke H, Seeber S, Vanhoefer U: Phase I study of a weekly schedule of infusional high-dose 5-fluorouracil/leucovorin/irinotecan in combination with biweekly cisplatin as first-line chemotherapy for metastatic gastric carcinoma. Proc Am Soc Clin Oncol, 2002 (Abstract #2259)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jefford, M., Michael, M., Rosenthal, M.A. et al. A Novel Combination of Cisplatin, Irinotecan, and Capecitabine in Patients with Advanced Cancer. Invest New Drugs 22, 185–192 (2004). https://doi.org/10.1023/B:DRUG.0000011796.20332.a9
Issue Date:
DOI: https://doi.org/10.1023/B:DRUG.0000011796.20332.a9