Abstract
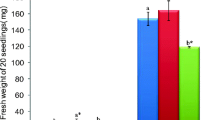
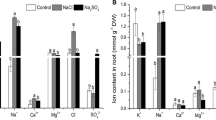
The effect of salt stress (100 mM and 200 mM NaCl) on antioxidant responses in shoots and roots of 14-day-old lentil (Lens culinaris M.) seedlings was investigated. Salt stress caused a significant decrease in length, wet-dry weight and an increase in proline content of both shoot and root tissues. In leaf tissues, high salinity treatment resulted in a 4.4 fold increase in H2O2 content which was accompanied by a significant level of lipid peroxidation and an increase in electrolyte leakage. Root tissues were less affected with respect to these parameters. Leaf tissue extracts exhibited four activity bands, of which two were identified as Cu/Zn-SOD and others as Fe-SOD and Mn-SOD. Fe-SOD activity was missing in root extracts. In both tissues Cu/Zn-SOD activity comprised 70–75% of total SOD activity. Salt stress did not cause a significant increase in total SOD activity of leaf tissues but a significant enhancement (88%) was observed in roots mainly due to an enhancement in Cu/ZnSOD isoforms. Compared to leaf tissues a significantly higher constitutive ascorbate peroxidase (APX) and glutathion reductase (GR) activity was observed in root tissues. Upon salt stress no significant change in the activity of APX, catalase (CAT) and GR was observed in root tissues but a higher APX activity was present when compared to leaf tissues. On the other hand, in leaf tissues, with the exception of CAT, salt stress caused significant enhancement in the activity of other antioxidant enzymes. These results suggested that, root tissues of lentil are protected better from NaCl stress induced oxidative damage due to enhanced total SOD activity together with a higher level of APX activity under salinity stress. To our knowledge this is the first report describing antioxidant enzyme activities in lentil.
Similar content being viewed by others
References
Asada K. 1999. The water-water cycle in chloroplast: scavenging of active oxygens and dissipation of excess photons. Ann. Rev. Plant Physiol. Plant Biol. 50: 601–639.
Ashraf M. and Waheed A. 1990. Screening of local/exotic accessions of lentil (Lens culinaris) for salt tolerance at two growth stages. Plant Soil 128: 167–176.
Bates L.S., Waldren R.P. and Teare I.D. 1977. Rapid determination of free proline for water stress studies. Plant Soil 39: 205–207.
Beauchamp C. and Fridovich I. 1971. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 44: 151–155.
Benavides M.P., Marconi P.L., Gallego S.M., Combo M.E. and Tomaro M.L. 2000. Relationship between antioxidant defense system and salt tolerance in Solanum tuberosum Aust. J. Plant Physiol. 27: 273–278.
Bernt E. and Bergmeyer H.U. 1974. Inorganic peroxidases. In: Bergmeyer H.U. (eds), Methods of Enzymatic Analysis, vol. 4, Academic Press, NY, pp. 2246–2248.
Biemelt S., Keetman U., Mock H.P. and Grimm B. 2000. Expression and activity of isozymes of superoxide dismutase in wheat roots in response to hypoxia and anoxia. Plant Cell Environ. 23: 135–144.
Bradford M.M. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254.
Bueno P., Piqueras A., Kurepa J., Savoure A., Verbruggen N., Montagu V.M. and Inze D. 1998. Expression of antioxidant enzymes in response to abscisic acid and high osmoticum in tobacco BY-2 cell cultures. Plant Sci. 138: 27–34.
Burke J.J., Melvin O.J. and Oliver M.J. 1992. Differential temperature sensitivity of pea superoxide dismutase. Plant Physiol. 100: 1–4.
Chance B. and Maehly A.C. 1955. Assay of catalases and peroxidases. Methods Enzymol. 2: 764–817.
Comba M.E., Benavides M.P., Gallego S.M. and Tomaro M.L. 1998. Relationship between nitrogen fixation and oxidative stress induction in nodules of salt treated soybean plants. Phyton-Int. J. Exp. Bot. 60: 115–126.
Dat J., Vandenabeele S., Vranova E., Van Montagu M., Inze D. and Van Breusegem F. 2000. Dual action of the active oxygen species during plant stress responses. Cell. Molec. Life Sci. 57: 779–795.
Delauney A.J. and Verma D.P.S. 1993. Proline biosynthesis and osmoregulation in plants. Plant J. 4: 215–223.
Dionisio-Sese M.L. and Tobita S. 1998. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 135: 1–9.
Fridovich I. 1982. Measuring the activity of superoxide dismutases: an embarrassment of riches. In: Oberly L.W. (ed.), Superoxide Dismutase, vol. 1, CRC Press, Boca Baton, pp. 69–77.
Ghoulam C., Foursy A. and Fares K. 2002. Effects of salt stress on growth, inorganic ions and proline accumulation in relation to osmotic adjustment in five sugar beet cultivars. Environ. Exp. Bot. 47: 39–50.
Gueta-Dahan Y., Yaniv Z., Zilinskas B.A. and Ben-Hayyim G. 1997. Salt and oxidative stress: similar and specific responses and their relation to salt tolerance in Citrus. Planta 203: 460–469.
Hernandez J.A., Almansa M.S., Del Rio L.A. and Sevilla F. 1993. Effect of salinity on metalloenzymes of oxygen metabolism in two leguminous plants. J. Plant Nutr. 16: 2539–2554.
Hernandez J.A., Campillo A., Jimenez A., Alarcon J.J. and Sevilla F. 1999. Responses of antioxidant systems and leaf water relations to NaCl stress in pea plants. New Phytol. 141: 241–251.
Hoagland D.R. and Arnon D.I. 1950. The water-culture method for growing plants without soil. Cal. Agric. Exp. Sta. Ciru. 347: 1–32.
Jimenez A., Hernandez J.A., del Rio L.A. and Sevilla F. 1997. Evidence for the presence of ascorbate-glutathion cycle in mitochondria and peroxisomes of pea (Pisum sativum L.) leaves. Plant Physiol. 114: 101–108.
Katerji N., van Hoorn J.W., Hamdy A., Mastrorilli M., Oweis T. and Erskine W. 2001. Response of two varieties of lentil to soil salinity. Agric. Water Manage. 47: 179–190.
Khan M.H., Singha K.L.B. and Panda S.K. 2002. Changes in antioxidant levels in Oryza sativa L. roots subjected to NaCl salinity stress. Acta Physiol. Plant. 24: 145–148.
Kurepa J., Herouart D., Van Mantagu M. and Inze D. 1997. Differential expression of CuZn and Fe-superoxide dismutase genes of tobacco during development, oxidative stress, and hormonal treatments. Plant Cell Physiol. 38: 463–470.
Kwiatowski J. and Kaniuga Z. 1984. Evidence for ironcontaining superoxide dismutase in leaves of Lycopersicon esculentum and Phaseolus vulgaris. Acta Physiol. Plant. 6: 197–202.
Laemmli U.K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685.
Lechno S., Zamski E. and Telor E. 1997. Salt stressinduced responses in cucumber plants. J. Plant Pysiol. 150: 206–211.
Lee D.H., Kim Y.S. and Lee C.B. 2001. The inductive responses of antioxidant enzymes by salt stress in rice (Oryza sativa L.). J. Plant Physiol. 158: 737–745.
Lin C.C. and Kao C.H. 2000. Effect of NaCl on H2O2 metabolism in rice leaves. Plant Growth Regul. 30: 151–155.
Mansour M.M.F. 1998. Protection of plasma membrane of onion epidermal cells by glycinebetaine and proline against NaCl stress. Plant Physiol. Biochem. 36: 767–772.
Meneguzzo S., Navario-Izzo F. and Izzo R. 1999. Antioxidative responses of shoots and roots of wheat to increasing NaCl concentrations. J. Plant Physiol. 155: 274–280.
Nanjo T., Kobayashi M., Yoshiba Y., Kakubar Y., Yamaguchi Shinozaki K. and Shinozaki K. 1999. Antisense suppression of the proline degradation improves tolerance to freezing and salinity in Arabidopsis thaliana. FEBS Lett. 461: 205–210.
Nieman R.H. 1965. Expansion of bean leaves and its suppression by salinity. Plant Physiol. 40: 156–161.
Ohkawa H., Ohishi N. and Yagi Y. 1979. Assay of lipid peroxides in animal tissue by thiobarbituric acid reaction. Anal. Biochem. 95: 51–358.
Sairam R.K. and Srivastava G.C. 2002. Changes in antioxidant activity in sub-cellular fractions of tolerant and susceptible wheat genotypes in response to long term salt stress. Plant Sci. 162: 897–904.
Santa-Cruz A., Perez-Alfocea F., Caro M. and Acosta M. 1998. Polyamines as short-term salt tolerance traits in tomato. Plant Sci. 138: 9–16.
Sgherri C.L.M., Liggini B., Puliga S. and Navari-Izzo F. 1994. Antioxidant system in Sporobolus stapfianus: changes in response to desiccation and rehydration. Phytochemistry 35: 561–565.
Sudhakar C., Lakshmi A. and Giridarakumar S. 2001. Changes in the antioxidant enzyme efficacy in two high yielding genotypes of mulberry (Morus alba L.) under NaCl salinity. Plant Sci. 161: 613–619.
Tramontano W.A. and Jouve D. 1997. Trigonelline accumulation in salt stressed legumes and the role of other osmoregulators as cell cycle control agents. Phytochemistry 44: 1037–1040.
Wang S.Y., Jiao H. and Faust M. 1991. Changes in ascorbate, glutathione and related enzyme activities during thiodiazuroninduced bud break of apple. Plant Physiol. 82: 231–236.
Yu Q. and Rengel Z. 1999. Drought and salinity differentially influence the activities of superoxide dismutases in narrowleafed lupins. Plant Sci. 142: 1–11.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bandeoğlu, E., Eyidoğan, F., Yücel, M. et al. Antioxidant responses of shoots and roots of lentil to NaCl-salinity stress. Plant Growth Regulation 42, 69–77 (2004). https://doi.org/10.1023/B:GROW.0000014891.35427.7b
Issue Date:
DOI: https://doi.org/10.1023/B:GROW.0000014891.35427.7b