Abstract
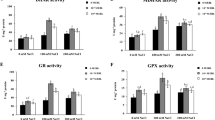
The effects of 24-epibrassinolide (24-epiBL) on seedling growth, antioxidative system, lipid peroxidation, proline and soluble protein content were investigated in seedlings of the salt-sensitive rice cultivar IR-28. Seedling growth of rice plants was improved by 24-epiBL treatment under salt stress conditions. When seedlings treated with 24-epiBL were subjected to 120 mM NaCl stress, the activities of superoxide dismutase (EC 1.15.1.1), catalase (EC 1.11.1.6) and glutathione reductase (EC 1.6.4.2) did not show significant difference, whereas the activity of ascorbate peroxidase (EC 1.11.1.11) significantly increased. Increased activity of peroxidase (EC 1.11.1.7) under NaCl stress showed remarkable decrease in the 24-epiBL+NaCl-applied group. Lipid peroxidation level significantly increased under salt stress but decreased with 24-epiBL application revealing that less oxidative damage occurred in this group (24-epiBL+NaCl). In addition, increased proline content in the NaCl-applied group was decreased by 24-epiBL application in the 24-epiBL+NaCl-applied group. Soluble protein content was increased by 24-epiBL application even under NaCl stress, being also higher than control conditions (no 24-epiBL or NaCl treatment). 24-epiBL treatment considerably alleviated oxidative damage that occurred under NaCl-stressed conditions and improved seedling growth in part under salt stress in sensitive IR-28 seedlings.
Similar content being viewed by others
References
Acar O., Türkan I. and Özdemir F. 2001. Superoxide dismutase and peroxidase activities in drought sensitive and resistant barley (Hordeum vulgare L.) varieties. Acta Physiol. Plant. 23(3): 351–356.
Anuradha S. and Rao S.S.R. 2003. Application of brassinosteroids to rice seeds (Oryza sativa L.) reduced the impact of salt stress on growth, prevented photosynthetic pigment loss and increased nitrate reductase activity. Plant Growth Regul. 40: 29–32.
Aono M., Saji H., Fujiyama K., Sugita M., Kondo N. and Tanaka K. 1995. Decrease in activity of glutathione reductase enhances paraquat sensitivity in transgenic Nicotiana tabacum. Plant Physiol. 107: 645–648.
Bajguz A. 2000a. Effect of brassinosteroids on nucleic acids and protein content in cultured cells of Chlorella vulgaris. Plant Physiol. Biochem. 38(3): 209–215.
Bajguz A. 2000b. Blockade of heavy metals accumulation in Chlorella vulgariscells by 24-epibrassinolide. Plant Physiol. Biochem. 38: 797–801.
Bates L.S., Waldren R.P. and Teare I.D. 1973. Rapid determination of free proline for water-stress studies. Plant Soil 39: 205–207.
Beauchamp C.O. and Fridovich I. 1971. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 44: 276–287.
Bergmeyer N. 1970. Methoden der enzymatischen Analyse, vol. 1, Akademie Verlag, Berlin, pp. 636–647.
Bor M., Özdemir F. and Türkan I. 2003. The effect of salt stress on lipid peroxidation and antioxidants in leaves of sugar beet Beta vulgaris L. and wild beet Beta maritimaL. Plant Sci. 164: 77–84.
Bradford M.M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254.
Clouse S.D., Langford M., Hall A.F., McMorris T.C. and Baker M.E. 1993. Physiological and molecular effects of brassinosteroids on Arabidopsis thaliana. J. Plant Growth Regul. 12: 61–66.
Dhaubhadel S., Chaudhary S., Dobinson K.F. and Krishna P. 1999. Treatment with 24-epibrassinolide, a brassinosteroid, increases the basic thermotolerance of Brassica napusand tomato seedlings. Plant Mol. Biol. 40: 333–342.
Dionisio-Sese M.L. and Tobita S. 1998. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 135: 1–9.
Foyer C.H. and Halliwell B. 1976. The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133: 21–25.
Fujioka S. 1999. Natural occurrence of brassinosteroids in the plant kingdom. In: Sakurai A., Yokota T. and Clouse S.D. (eds), Brassinosteroids: Steroidal Plant Hormones, Springer-Verlag, Tokyo, pp. 21–45.
Fujioka S. and Sakurai A. 1997. Brassinosteroids. Nat. Prod. Rep. 14: 1–10.
Grove M.D., Spencer G.F., Rohwedder W.K., Mandava N.B., Worley J.F., Warthen J.D., Steffens G.L., Flippen-Anderson J.L. and Cook J.C. 1979. Brassinolide, a plant growthpromoting steroid isolated from Brassica napuspollen. Nature 281: 216–217.
Hasegawa P.M., Bressan R.A., Zhu J.K. and Bohnert H.J. 2000. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 51: 463–499.
Hernández J.A., Jiménez A., Mullineaux P. and Sevilla F. 2000. Tolerance of pea (Pisum sativum L.) to long-term salt stress is associated with induction of antioxidant defenses. Plant Cell Environ. 23: 853–862.
Herzog V. and Fahimi H. 1973. Determination of the activity of peroxidase. Anal. Biochem. 55: 554–562.
Jain M., Mathur G., Koul S. and Sarin N.B. 2001. Ameliorative effects of proline on salt stress-induced lipid peroxidation in cell lines of groundnut (Arachis hypogea L.). Plant Cell Rep. 20: 463–468.
Li J.M., Nagpal P., Vitart V., Mc Morris T.C. and Chory J. 1996. A role for brassinosteroids in light dependent development of Arabidopsis. Science 272: 398–401.
Li L. and Van Staden J. 1998. Effects of plant growth regulators on drought resistance of two maize cultivars. S. Afr. J. Bot. 64(2): 116–120.
Li L., Van Staden J. and Jäger A.K. 1998. Effects of plant growth regulators on the antioxidant system in seedlings of two maize cultivars subjected to water stress. Plant Growth Reg. 25: 81–87.
Lin J.N. and Kao C.H. 1998. Effect of oxidative stress caused by hydrogen peroxide on senescence of rice leaves. Bot. Bull. Acad. Sin. 39: 161–165.
Madhava Rao K.V. and Sresty T.V.S. 2000. Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan L. Millspaugh) in response to Zn and Ni stresses. Plant Sci. 157: 113–128.
Mandava N.B. 1988. Plant growth-promoting brassinosteroids. Annu. Rev. Plant Physiol. Plant Mol. Biol. 39: 23–52.
McCord J.M. 2000. The evolution of free radicals and oxidative stress. Am. J. Med. 108: 652–659.
Mittal R. and Dubey R.S. 1991. Behaviour of peroxidases in rice: changes in enzyme activity and isoforms in relation to salt tolerance. Plant Physiol. Biochem. 29(1): 31–40.
Nakano Y. and Asada K. 1981. Hydrogen peroxide is scavenged by ascorbate-spesific peroxidase in spinach chloroplasts. Plant Cell Physiol. 22(5): 867–880.
Noctor G. and Foyer C.H. 1998. Ascorbate and glutathione: Keeping active oxygen under control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49: 249–279.
Nomura T., Nakayama N., Reid J.B., Takeuchi Y. and Yokota T. 1997. Blockage of brassinosteroid biosynthesis and sensitivity cause dwarfism in Pisum sativum. Plant Physiol. 113: 31–37.
Sasse J.M. 1997. Recent progress in brassinosteroid research. Physiol. Plant. 100: 696–701.
Sasse J.M. 1999. Physiological actions of brassinosteroids. In: Sakurai A., Yokota T. and Clouse S.D. (eds), Brassinosteroids: Steroidal plant hormones, Springer-Verlag, Tokyo, pp. 137–161.
Sasse J.M., Smith R. and Hudson I. 1995. Effects of 24-epibrassinolide on germination of seed of Eucalyptus camaldulensisin saline conditions. Proc. Plant Growth Regul. Soc. Amer. 22: 136–141.
Schumacher K. and Chory J. 2000. Brassinosteroid signal transduction: still casting the actors. Curr. Opin. Plant Biol. 3: 79–84.
Singha S. and Choudhuri M.A. 1990. Effect of salinity (NaCl) stress on H2O2 metabolism in Vigna and Oryza seedlings. Biochem. Physiol. Pflanzen 186: 69–74.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Özdemir, F., Bor, M., Demiral, T. et al. Effects of 24-epibrassinolide on seed germination, seedling growth, lipid peroxidation, proline content and antioxidative system of rice (Oryza sativa L.) under salinity stress. Plant Growth Regulation 42, 203–211 (2004). https://doi.org/10.1023/B:GROW.0000026509.25995.13
Issue Date:
DOI: https://doi.org/10.1023/B:GROW.0000026509.25995.13