Abstract
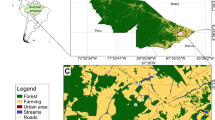
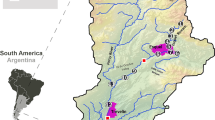
Assessment of the environmental factors that control species richness (S) is a central issue in ecology. In this study, aquatic macrophyte S was estimated in 235 sampling sites distributed in 8 arms of a large (1350 km2) subtropical reservoir (Itaipu Reservoir, Brazil). Morphometric variables (area, shoreline development and length of shoreline, all measured for each arm; n= 8) and environmental variables measured at each sampling site (extinction coefficient of light (k), electrical conductivity, fetch, distance from the main reservoir body; n = 235) were used to predict aquatic macrophyte S at two spatial scales. At arm scale, linear regression analysis indicated that length of shoreline was a better predictor of S than area. At sampling site scale, multiple regression analysis indicated that S was significantly predicted by electrical conductivity, fetch and distance from the main body. However, other relationships with predictive interest was demonstrated by using non-traditional regression approaches. This analysis started by the visual inspection of scatter plots. The bivariate relationship between S and fetch, for example, showed an envelope or a `left triangle' pattern. The relationship between the number of submerged species and k showed an asymmetrical left triangle pattern. Using randomization procedures, it was demonstrated that these patterns were not generated by chance alone. Beta diversity (estimated within the arms) was significantly and positively correlated with spatial environmental variability. Overall, these results indicate that the prediction of aquatic macrophytes assemblage variables in large waterbodies, specially S, is more complex than previous studies have suggested.
Similar content being viewed by others
References
Agostinho, A. A., A. E. A. Vazzoler & S. M. Thomaz, 1995. The High Paraná River Paraná basin: limnological and ichthyological aspects. In Tundisi, J. G. C. E. M. Bicudo & T. Matsumura-Tundisi (eds), Limnology in Brazil. Brazilian Academy of Science/Brazilian Society of Limnology, Rio de Janeiro: 59–104.
Agostinho, A. A., L. E. Miranda, L. M. Bini, L. C. Gomes, S. M. Thomaz & H. I. Suzuki, 1999. Patterns of colonization in neotropical reservoirs, and prognosis on aging. In Tundisi J. G. & M. Stra¢skraba (eds), Theoretical Reservoir Ecology and its Applications. International Institute of Ecology/Brazilian Academy of Sciences/Backhuys Publishers, São Carlos: 227–265.
Agostinho, A. A. & L. C. Gomes, 1997. Manejo e monitoramento de recursos pesqueiros: perspectivas para o reservatório de Segredo. In Agostinho A. A. & L. C. Gomes (eds), Reservatório de Segredo: Bases Ecológicas Para o Manejo. Eduem, Maringá: 319–264.
Amoros, C. & G. Bornette, 1999. Antagonistic and cumulative effects of connectivity: a predictive model based on aquatic vegetation in riverine wetlands. Arch. Hydrobiol. 3: 311–327.
Bini, L. M., S. M. Thomaz, K. J. Murphy & A. F. M. Camargo, 1999. Aquatic macrophyte distribution in relation to water and sediment conditions in the Itaipu Reservoir, Brazil. Hydrobiologia 415: 147–154.
Bini, L. M., S. M. Thomaz & D. C. Souza, 2001. Species richness and â-diversity of aquatic macrophytes in the Upper Paraná River floodplain. Arch. Hydrobiol. 151: 511–525.
Blackburn, T. M. & K. J. Gaston, 1996. The distribution of bird species in the New World: patterns in species turnover. Oikos 77: 146–152.
Bornette, G., H. Piegay, A. Citterio, C. Amoros & V. Godreau, 2001. Aquatic plant diversity in four river floodplains: a comparison at two hierarchical levels. Biodivers. Conserv. 10: 1683–1701.
Brown, J. H, 1995. Macroecology. University of Chicago Press, Chicago, pp.
Chambers, P. A., 1987. Nearshore occurrence of submersed aquatic macrophytes in relation to wave action. Can. J. Fish. aquat. Sci 44: 1666–1669.
Coops, H., R. Boeters & H. Smit, 1991. Direct and indirect effects of wave attack on helophytes. Aquat. Bot. 41: 333–352.
Coops, H. & R.W. Doef, 1996. Submerged vegetation development in two shallow, eutrophic lakes. Hydrobiologia 340: 115–120.
Duarte, C. M. & J. Kalff, 1986. Littoral slope as a predictor of the maximum biomass of submerged macrophyte communities. Limnol. Oceanogr. 31: 1072–1080.
Duarte, C. E., 1990. Variance and the description of nature. In Cole J. J., G. Lovet & S. Findlay (eds), Comparative Analyses of Ecosystems: Patterns, Mechanisms, and Theories. Springer Verlag, New York: 301–318.
Duarte, C. M., J. Kalff & R. H. Peters, 1986. Patterns in biomass and cover of aquatic macrophytes in lakes. Can. J. Fish. aquat. Sci. 43: 1900–1908.
Esteves, F. A. & R. Barbieri, 1983. Dry weight and chemical changes during decomposition of tropical macrophytes in Lobo Reservoir – Sáo Paulo, Brazil. Aquat. Bot. 16: 285–295.
Engelhardt, K. A. M. & M. E. Ritchie, 2001. Effects of macrophyte species richness on wetland ecosystem functioning and services. Nature 411: 687–689.
Gasith, A. & M. V. Hoyer, 1998. Structuring role of macrophytes in lakes: changing influence along lake size and depth gradients. In Jeppesen E., M. Søndergaard & K. Christoffersen (eds), The Structuring Role of SubmergedMacrophytes in Lakes. Ecological Studies, vol. 131. Springer, New York: 381–392.
Goldberg, D. E. & S. M. Scheiner, 1993. ANOVA and ANCOVA: Field competition experiments. In Scheiner S. M. & J. Gurevitch (eds), The Design and Analysis of Ecological Experiments. Chapman & Hall, New York: 69–93.
Gotelli, N. J. & G. L. Entsminger, 2000. EcoSim: Null models software for ecology. Version 5.0. Acquired Intelligence Inc. & Kesey-Bear. http://homepages.together.net/gentsmi n/ecosim.htm.
Håkanson, L. & M. Jansson, 1983. Principles of lake sedimentology. Springer-Verlag, Berlin. 316 pp.
Harrison, S., S. J. Ross & J. H. Lawton, 1992. Beta diversity on geographic gradients in Britain. J. anim. Ecol. 61: 151–158.
Heegaard, E., H. H. Birks, C. E. Gibson, S. J. Smith & S. Wolfe-Murphy, 2001. Species-environmental relationships of aquatic macrophytes in Northern Ireland. Aquat. Bot. 70: 175–223.
Henderson, H. V. & P. F. Velleman., 1981. Building multipleregression models interactively. Biometrics 37: 391–411.
Hudon, C., S. Lalonde & P. Gagnon, 2000. Ranking the effects of site exposure, plant growth form, water depth, and transparency on aquatic plant biomass. Can. J. Fish. aquat. Sci. 57: 31–42.
Jacobsen, D. & E. Terneus, 2001. Aquatic macrophytes in cool aseasonal and seasonal streams: a comparison betweenEcuadorian highland and Danish lowland streams. Aquat. Bot. 71: 281–295.
Jackson, S. T. & D. F. Charles, 1988. Aquatic macrophytes in Adirondack (New York) lakes: patterns of species composition in relation to environment. Can. J. Bot. 66: 1449–1460.
Kimmel, B. L., O. T. Lind & L. J. Paulson, 1990. Reservoir Primary Production. In Thornton K. W., B. L. Kimmel & F.E. Payne (eds), Reservoir Limnology: Ecological Perspectives. John Wiley & Sons, New York: 133–194.
Lougheed, V. L., B. Crosbie & P. Chow-Fraser, 2001. Primary determinants of macrophyte community structure in 62 marshes across the Great Lakes basin: latitude, land use, and water quality effects. Can. J. Fish. aquat. Sci. 58: 1603–1612.
Manly, B. F. J., 1991. Randomization and Monte Carlo Methods in Biology. Chapman and Hall, London. 281 pp.
Martínez, J. C. C., A. Canesin & C. C. Bonecker, 2000. Species composition of rotifers in different habitats of an artificial lake, Mato Grosso do Sul State, Brazil. Acta Scientiarum 22: 343–346.
Moore, J. A., 1986. Charophytes of Great Britain and Ireland. Botanical Society of the British Islands, London. 141 pp.
Murphy, K. J., B. Rørslett & I. S pringuel, 1990. Strategy analysis of submerged lake macrophyte communities: an international example. Aquat. Bot. 36: 303–323.
Nilsson, C. & P. A. Keddy, 1988. Predictability of change in shoreline vegetation in a hydroelectric reservoir, Northern Sweden. Can. J. Fish. aquat. Sci. 45: 1896–1904.
Rea, T. E., D. J. Karapatakis, K. K. Guy, J. E. Pinder III & H. E. Mackey Jr., 1998. The relative effects of water depth, fetch and other physical factors on the southeastern U.S. pond. Aquat. Bot. 61: 289–299.
Robach, F., I. Eglin & M. Tremolieres, 1997. Species richness of aquatic macrophytes in former channels connected to a river: a comparison between two fluvial hydrosystems differing in their regime and regulation. Global Ecol. Biogeogr. 6: 267–274.
Rørslett, B., 1991. Principal determinants of aquatic macrophyte richness in Northern European lakes. Aquat. Bot. 39: 173–193.
Smart, R. M., R. D. Doyle & J. D. Madsen, 1996. Establishing native submersed aquatic plant communities for fish habitat. Am. Fish. Soc. Symp. 16: 347–356.
Sand-Jensen, K., T. Riis, O. Vestergaard & S. E. Larsen, 2000. Macrophyte decline in Danish lakes and streams over the past 100 years. J. Ecol. 88: 1030–1040.
Scheffer, M., M. R. Redelijkheid & F. Noppert, 1992. Distribution and dynamics of submerged vegetation in a chain of shallow eutrophic lakes. Aquat. Bot. 42: 199–216.
Sokal, R. R. & F. J. Rohlf, 1981. Biometry. 2nd. Ed.W. H. Freeman and Company, New York. 859 pp.
Thomaz, S. M., L. M. Bini & D. C. Souza, 1998. Biomass and maximum colonization depth of Egeria najas Planchon (Hydrocharitaceae) at Itaipu Reservoir, Brazil. In Monteiro A., T. Vasconcelos & L. Catarino (eds), Management and Ecology of Aquatic Plants. Proceedings of the 10th EWRS International Symposium on Aquatic Weeds. EWRS/APRH, Lisbon: 223–226.
Thomaz, S. M. & L. M. Bini, 1999. A expansão das macrófitas aquáticas e implicações para o manejo de reservatórios: um estudo na represa de Itaipu. In Henry R. (ed), Ecologia de Reservatórios: Estrutura, Função e Aspectos Sociais. Fundibio, Botucatu: 599–625.
Thomaz, S.M., L. M. Bini, M. C. Souza, K.K. Kita & A. F. M. Camargo, 1999. Aquatic macrophytes of Itaipu Reservoir, Brazil: Survey of species and ecological considerations. Brazil. archiv. biol. technol. 42: 15–22.
Thornton, K. W., 1990. Sedimentary processes. In Thornton K. W., B. L. Kimmel & F. E. Payne (eds), Reservoir Limnology: Ecological Perspectives. John Wiley & Sons, New York: 43–69.
Tockner, K., F. Schiemer, C. Baumgartner, G. Kum, E. Weigand, I. Zweimuller & J. V. Ward, 1999. The Danube restoration project: Species diversity patterns across connectivity gradients in the floodplain system. Regul. Riv. 15: 245–258.
Vestergaard, O. & K. Sand-Jensen, 2000a. Aquatic macrophyte richness in Danish lakes in relation to alkalinity, transparency, and lake area. Can. J. Fish. aquat. Sci. 57: 2022–2031.
Vestergaard, O. & K. Sand-Jensen, 2000b. Alkalinity and trophic state regulate aquatic plant distribution in Danish lakes. Aquat. Bot. 67: 85–107.
Wall, D., H. Mooney, G. Adams, G. Boxshall, A. Dobson, T. Nakashizuka, J. Seyani, C. Samper & J. Sarukhan, 2001. An International Biodiversity Observation Year. Trends Ecol. Evol. 16: 52–54.
Whittaker, R. H., 1972. Evolution and measurement of species diversity. Taxon 21: 213–251.
Willby, N. J., J. R. Pygott, & J. W. Eaton, 2001. Inter-relationship between standing crop, biodiversity and trait attributes of hydrophytic vegetation in artificial waterways. Freshwat. Biol. 46: 883–902.
Wilson, M. V. & A. Shmida, 1984. Measuring beta diversity with presence-absence data. J. Ecol. 72: 1055–1064.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Thomaz, S.M., Souza, D.C. & Bini, L.M. Species richness and beta diversity of aquatic macrophytes in a large subtropical reservoir (Itaipu Reservoir, Brazil): the influence of limnology and morphometry. Hydrobiologia 505, 119–128 (2003). https://doi.org/10.1023/B:HYDR.0000007300.78143.e1
Issue Date:
DOI: https://doi.org/10.1023/B:HYDR.0000007300.78143.e1