Abstract
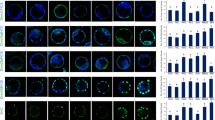
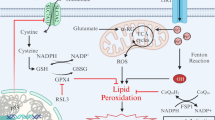
Oxidative stress plays an important role in tissue damage caused by hypoglycemia and diabetes, which may be the result of deterioration in glucose homeostasis caused by these metabolic disorders. The present study examined the effects of insulin-induced hypoglycemia and streptozotocin induced diabetes on mitochondrial lipid peroxidation and antioxidant enzymes from different brain regions, namely, cerebral hemispheres, cerebellum, brain stem and diencephalon. In situ localization of DNA single strand breaks (SSBs) were also studied by DNA polymerase-I mediated biotin dATP labeled nick translation method after inducing hypoglycemia and diabetes. Significant decrease in mitochondrial catalase, manganese superoxide-dismutase (Mn-SOD) and reduced glutathione (GSH) content and increase in the lipid peroxidation (LPx) and glutathione peroxidase (GPx) activity was observed under these metabolic stress conditions with more pronounced effects in hypoglycemic group. We conclude that during severe energy deprivation following hypoglycemia and diabetes, mitochondrial free radicals scavenger system is down regulated, which leads to reactive oxygen species (ROS) generation. High levels of ROS in turn activate the processes leading to DNA damage. DNA SSBs, which indicates nuclear disintegration is an important feature of neuronal cell death.
Similar content being viewed by others
References
Moley KH, Mueckler MM: Glucose and apoptosis. Apoptosis 5: 99-105, 2000
Siesjo BK: Hypoglycemia, brain metabolism, and brain damage. Diabetes Metab Rev 4: 113-144, 1988
Erecinska M, Silver IA: ATP and brain function. J Cereb Blood Flow Metab 9: 2-19, 1989
Nellgard B, Weloch T: NMDA-receptor blockers but not NBQX, an AMPA-receptor antagonist, inhibit spreading depression in the rat brain. Acta Physiol Scand 146: 497-503, 1992
Choi DW: Glutamate neurotoxicity and diseases of the nervous system. Neuron 1: 623-634, 1988
Coyle JT, Puttfarcken P: Oxidative stress, glutamate, and neuro-degenerative disorders. Science 262: 689-695, 1993
Nedergaard M: Transient focal ischemia in hyperglycemic rats is associated with increased with cerebral infarction. Brain Res 408: 79-85, 1987
Lundgren J, Smith ML, Siesjo BK: Effects of dimethylthiourea on ischemic brain damage in the hyperglycemic rat. J Neurol Sci 113: 187-197, 1992
Li PA, Gisselsson L, Keuker J, Vogel J, Smith ML, Kuschinsky W, Siesjo BK: Hyperglycemia-exaggerated ischemic brain damage following 30 min of middle cerebral artery occlusion is not due to capillary obstruction. Brain Res 804: 36-44, 1998
Brownlee M Cerami A, Vlassara H: Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med 28: 1315-1321, 1988
Koya D, King GL: Protein kinase C activation and the development of diabetic complications. Diabetes 17: 859-866, 1998
Siesjo BK, Katsura KI, Zhao Q, Folbergrova J, Pahlmark K, Siesjo P, Smith M: Mechanism of secondary brain damage in global and focal ischemia: A speculative synthesis. J. Neurotrauma 12: 943-956, 1995
Ferrand-Drake M, Friberg H, Wieloch T: Mitochondrial permeability transition induced DNA fragmentation in the rat hippocampus following hypoglycemia. Neuroscience 90: 1325-1338, 1999
Nomura K, Imai H, Koumura T, Arai M, Nakagawa Y: Mitochondrial phospholipid hydroperoxide glutathione peroxidase suppresses apoptosis mediated by a mitochondrial death pathway. J Biol Chem 274: 29294-29302, 1999
Manna SK, Zhang HJ, Tao Y, Oberley LW, Aggarwal BB: Overexpression of manganese superoxide dismutase suppresses tumor necrosis factor-induced apoptosis and activation of nuclear transcription factor-kB and activated protein-1. J Biol Chem 273: 13245-13254, 1998
Chen J, Jin K, Minzhi C, Pei W, Kawaguchi K, Greenberg DA, Simon RP: Early detection of DNA strand breaks in the brain after transient focal ischemia: Implication for the role of DNA damage in apoptosis and neuronal cell death. J Neurochem 69: 232-245, 1997
Didier M, Bursztain S, Adamec E, Passani L, Nixon RA, Coyle JT, Wei JY, Berman SA: DNA strand breaks induced by sustained glutamate excitotoxicity in primary neuronal cultures. J Neurosci 16: 2238-2250, 1996
Bhardwaj SK, Shrama P, Kaur G: Alterations in free radical scavenger system profile of type I diabetic rat brain. Mol Chem Neuropathol 35: 187-202, 1998
Bhardwaj SK, Sharma ML, Gulati G, Chhabra A, Kaushik R, Sharma P, Kaur G: Effect of starvation and insulin-induced hypoglycemia on oxidative stress scavenger system and electron transport chain complexes from rat brain, liver, and kidney. Mol Chem Neuropathol 34: 157-168, 1998
Kaur G, Bhardwaj SK: The impact of diabetes on CNS. Role of bioenergetic defects. Mol Chem Neuropathol. 35: 119-131, 1998
McCord JM, Fridovich I: Superoxide dismutase: An enzymatic function for erythrocuprein (hemocuprein). J Biol Chem 244: 6049-6055, 1969
Aebi H: Catalase in vitro. In: L. Packer (ed). Methods in Enzymology. Academic Press, New York, 1984, pp 121-126
Sedlak J, Lindsay RH: Estimation of total, protein bound and non-protein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem 25: 192-205, 1968
Flohe L, Gunzler WA: Assays of glutathione peroxidase. In: L. Packer (ed). Methods in Enzymology. Academic Press, New York, 1984, pp 114-121
Beuge JA, Aust AD: Microsomal lipid peroxidation. Meth Enzymol 52: 302-310, 1978
Iseki S: DNA damage and oxygen radical toxicity. Science 240: 1302-1309, 1986
Zhu CH, Huang Y, Oberley LW, Domann FE: A family of AP-2 proteins down-regulate manganese superoxide dismutase expression. J Biol Chem 276: 14407-14413, 2001
Morel Y, Barouki R: Repression of gene expression by oxidative stress. Biochem J 342: 481-496, 1999
Halliwell B, Aruoma OI: DNA damage by oxygen derived species. Its mechanism and measurement in mammalian system. FEBS Lett 281: 9-19, 1991
Bai J, Rodriguez AM, Melendez JA, Cederbaum AI: Overexpression of catalase in cytosolic or mitochondrial compartment protects Hep G2 cells against oxidative injury. ?Journal, vol, page nos? 1999
Nomura K, Ima H, Koumura T, Kobayashi T, Nakagawa Y: Mitochondrial phospholipid hydroperoxide glutathione peroxidase inhibits the release of cytochrome c from mitochondria by suppressing the peroxidation of cardiolipin in hypoglycemia-induced apoptosis. Biochem J 351: 183-193, 2000
Zoratti M, Szabo I: The mitochondrial permeability transition. Biochem Biophys Acta 1241: 139-176, 1995
Burdon RH: Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radic Biol Med 18: 775-794, 1995
Michiels C, Raes M, Toussaint O, Remacle J: Importance of Se-glutathione peroxidase, catalase, and Cu/Zn-SOD for cell survival against oxidative stress. Free Radic Biol Med 17: 235-248, 1994
Henle ES, Linn S: Formation, prevention, and repair of DNA damage by iron/hydrogen peroxide. J Biol Chem 272: 19095-19098, 1997
Dringen R, Hamprecht B: Involvement of glutathione peroxidase and catalase in the disposal of exogenous hydrogen peroxide by cultured astroglial cells. Brain Res 759: 67-75, 1997
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Singh, P., Jain, A. & Kaur, G. Impact of hypoglycemia and diabetes on CNS: Correlation of mitochondrial oxidative stress with DNA damage. Mol Cell Biochem 260, 153–159 (2004). https://doi.org/10.1023/B:MCBI.0000026067.08356.13
Issue Date:
DOI: https://doi.org/10.1023/B:MCBI.0000026067.08356.13