Abstract
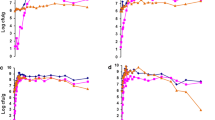
Balls of cowpea-daddawa, contaminated with Salmonella typhimurium (LT2) during processing, were exposed to sun drying (33–35 °C) for 3 days and smoking (60–80 °C) for the same period. On the first day, the initial contaminant level for both sun dried and smoked cowpeadaddawa was 104 cfu/g. Contaminant levels fell from 104 to 102 cfu/g on the second day of smoking, while the contaminants were no longer detected on the third day. The reduction in contaminant level for sun drying was a gradual process. It reduced from 104cfu/g on the first day to 102 cfu/g on the third day. Similarly, other organisms associated with daddawa fermentation could not survive beyond the first day of smoking. It is possible therefore that smoking and the sublethal heat injury of S. typhimurium, could be an effective way of eliminating bacterial pathogens from fermented processed daddawa.
Similar content being viewed by others
References
Antai SP, Ibrahim MH (1986) Microorganisms associated with African locust bean (Parkia filicoidea) fermentation for dawadawa production. Journal of Applied Bacteriology, 61: 145–148.
Barimalaa IS, Achinewhu SC, Yibatama I, Amadi EN (1994) Studies on the solid substrate fermentation of bambara groundnut (Vignea subterranea). Journal of the Science of Food and Agriculture 66: 443–446.
Buchannan RC, Gibbons NE (1974) Bergey's Manual of Determinative Bacteriology, Baltimore, Waverly Press Inc.
Collins C, Lyle PM (1984) Microbiology Methods. (8th edn) London, Butteworths.
Frazier WC, Westhoff DC (1973) Food Microbiology. Pp 144–145. New Delhi, Tata McGraw Hill.
Gomez G, Valdivieso D, Dela Cuesta D, Kawano K (1984) Cyanide content in wholeroot chips of ten cassava cultivars and its reduction by oven drying or sun drying on trays. Journal of Food Technology 19: 97–102.
Handford PM, Gibbs BM (1964) Antibacterial effects of smoke constituents on bacteria isolated from bacon. In Microbial inhibitors in food. Stockholm Almquist, Wiskell.
Henry DP, Frost AJ, Samuel JL, Boyle DAO, Thomson RH (1983) Factors affecting the survival of Salmonella and Escherichia coli in anaerobically fermented pig waste. Journal of Applied Bacteriology 55: 89–95.
Ihekoronye AI, Ngoddy PO (1985) Integrated Food Science and Technology for the Tropics pp 290–291. New York, Macmillan International College.
Jay JM (1987) Modern Food Microbiology. 3rd edn pp 287–288. Delhi India CBS Publishers and Distributors.
Njoku HO, Okwelle AA (1992) Storage stability of fermented African oil bean as affected by chemical preservative methods. In Traditional African Foods-Quality and Nutrition pp 95–98 (edited by A Westby, PJA Reilly) Stockholm, Sweden, International Foundation for Science.
Njoku HO, Ogbulie JN, Nnubia CO (1990) Microbiological study of the traditional processing of African oil bean (Pentaclethra macrophulla Bentham) for ugba production. Food Microbiology 7: 13–26.
Odunfa SA (1986) Dawadawa In: Legume Based Fermented Foods (edited by NR Reddy, MD Person, DK Salunke) pp 173–189 Boa Katon, Florida: CRC Press.
Odunfa SA, Komolafe OB, Ekunsanmi, JT (1987) Effect of chemical preservatives on the microorganisms isolated from fermenting African locust bean. Nigerian Food Journal 5: 66–75.
Ogbadu L, Okagbue RN (1988) Bacterial fermentation of soya bean for daddawa production. Journal of Applied Bacteriology 65: 353–356.
Steinkraus, KH (1992) Alkaline fermented foods and their relation to similar foods in other parts of the world. In: Traditional African Foods-Quality and Nutrition pp 87–92 (edited by A Westby PJA Reilly) Stockholm, Sweden, Intenational foundation for Science.
Twiddy DR, Cross DJ, Cooke RD (1987) Parameters involved in the production of lactic acid pre-served fish-starchy substrate combinations. International Journal of Food science and Technology 22: 115–121.
Wachukwu CK (1998) Production by Process and Substrate Modification of Ugba and Daddawa Two Alkaline Fermented Nigerian food condiments. PhD Thesis, Rivers State University of Science and technology, Port Harcourt, Nigeria.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wachukwu, C., Sokari, T. & Wemedo, S. Effects of sun drying and smoking on Salmonella typhimurium (LT2) during cowpea-daddawa processing. Plant Foods Hum Nutr 58, 1–7 (2003). https://doi.org/10.1023/B:QUAL.0000041145.43684.2f
Issue Date:
DOI: https://doi.org/10.1023/B:QUAL.0000041145.43684.2f