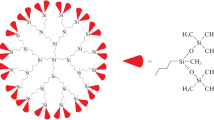
Abstract
A universal method for the synthesis of hydrophilic dendrimers was considered. The method is based on a combination of carbosilane dendrimers with different molecular organizations and hydrophilizing agents, viz., substituted hydride silanes containing one and three protected hydroxyl groups. The combination of a limited set of the mentioned reagents makes it possible to control the ratio of hydrophilic and hydrophobic moieties of the molecular structure in wide limits. A simple and convenient method for the removal of trimethylsilyl protection of hydroxyl groups in the surface layer of dendrimers was developed.
Similar content being viewed by others
References
L. Dai, J. Lu, B. Matthews, and A. W. H. Mau, J. Phys. Chem. B, 1998, 102, 4049.
M. Liu, K. Kono, and J. M. J. Frechet, J. Polym. Sci., Part A, 1999, 37, 3492.
Y. Pan and W. T. Ford, Macromolecules, 2000, 33, 3731.
S.-E. Stiriba, H. Frey, and R. Haag, Angew. Chem., 2002, 114, 1385.
H. Frey and R. Haag, Rev. Mol. Biotechnol., 2002, 90, 257.
L.-L. Zhou and J. Roovers, Macromolecules, 1993, 26, 963.
C. Kim, E. Park, and E. Kang, Bull. Korean Chem. Soc., 1996, 17, 592.
S. W. Krska and D. Seyferth, J. Am. Chem. Soc., 1998, 120, 3604.
K. Lorenz, R. Mülhaupt, H. Frey, U. Rapp, and F. J. Mayer-Posner, Macromolecules, 1995, 28, 6657.
C. Kim and S.-K. Choi, Main Group Met. Chem., 1997, 20, 143.
D. K. Polyakov, G. M. Ignat'eva, E. A. Rebrov, N. G. Vasilenko, S. S. Sheiko, M. Möller, and A. M. Muzafarov, Vysokomol. Soedin., Ser. A, 1998, 40, 1421 [Polym. Sci., Ser. A, 1998, 40, 876 (Engl. Transl.)].
S. A. Ponomarenko, E. A. Rebrov, N. I. Boiko, A. M. Muzafarov, and V. P. Shibaev, Vysokomol. Soedin., Ser. A, 1998, 40, 1253 [Polym. Sci., Ser. A, 1998, 40, 763 (Engl. Transl.)].
C. Kim, K. Jeong, and I. Jung, J. Polym. Sci., Part A, 2000, 38, 2749.
H. Frey, K. Lorenz, R. Mülhaupt, U. Rapp, and F. J. Mayer-Posner, Macromol. Symp., 1996, 102, 19.
E. V. Getmanova, T. B. Chenskaya, O. B. Gorbatsevich, E. A. Rebrov, N. G. Vasilenko, and A. M. Muzafarov, React. Funct. Polym., 1997, 33, 289.
G. M. Ignat'eva, E. A. Rebrov, V. D. Myakushev, M. N. Il'ina, I. I. Dubovik, and V. S. Papkov, Vysokomol. Soedin., Ser. A, 1997, 39, 1302 [Polym. Sci., Ser. A, 1997, 39, 874 (Engl. Transl.)].
N. G. Vasilenko, E. A. Rebrov, A. M. Muzafarov, B. Eβwein, B. Strieget, and M. Möller, Macromol. Chem. Phys., 1998, 199, 889.
E. V. Getmanova, E. A. Rebrov, N. G. Vasilenko, and A. M. Muzafarov, Polym. Prep., 1998, 39, 581.
B. A. Omotowa, K. D. Keefer, R. L. Kirchmeier, and J. M. Shreeve, J. Am. Chem. Soc., 1999, 121, 11130.
C. Kim, S. Son, and B. Kim, J. Organomet. Chem., 1999, 588, 1.
E. V. Getmanova, E. A. Rebrov, V. D. Myakushev, T. B. Chenskaya, M. J. Krupers, and A. M. Muzafarov, Vysokomol. Soedin., Ser. A, 2000, 42, 943 [Polym. Sci., Ser. A, 2000, 42, 610 (Engl. Transl.)].
K. Aoi, K. Iton, and M. Okada, Macromolecules, 1995, 28, 5391.
P. R. Ashton, E. F. Hounsell, N. Jayaraman, T. M. Nilsen, N. Spencer, J. F. Stoddart, and M. Young, J. Org. Chem., 1998, 63, 3429.
G. M. Pavlov, E. V. Korneeva, K. Jumel, S. E. Harding, E. W. Meijer, H. W. I. Peerlings, J. F. Stoddart, and S. A. Nepogodiev, Carbohydr. Polym., 1999, 38, 195.
R. Roy, M.-G. Baek, and K. Rittenhouse-Olson, J. Am. Chem. Soc., 2001, 123, 1809.
J. K. Young, G. R. Baker, and G. R. Newkome, Macromolecules, 1994, 27, 3464.
R. C. van Duijvenbode, A. Rajanayagam, G. J. M. Koper, M. W. P. L. Baars, B. F. M. de Waal, E. W. Meijer, and M. Borcovec, Macromolecules, 2000, 33, 46.
V. Bazant, V. Chvalovsky, and J. Rathousky, Organosilicon Compounds, Czechoslovak Academy of Sciences, Prague, 1965, 1, 57.
M. G. Voronkov, V. P. Mileshkevich, and Yu. A. Yuzhelevskii, Siloksanovaya svyaz' [Siloxane Bond], Nauka, Novosibirsk, 1976, 284 (in Russian).
L. N. Lewis, R. E. Colborn, H. Grade, G. L. Bryant, C. A. Sumpter, and R. A. Scott, Organometallics, 1995, 14, 2202.
R. Reimschneider and H. J. Kotzch, Monatsh. Chem., 1959, 90, 787.
R. Evans and J. A. Gallaghan, J. Am. Chem. Soc., 1953, 75, 1248.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Getmanova, E.V., Tereshchenko, A.S., Ignat"eva, G.M. et al. Diphilic carbosilane dendrimers with different densities of the hydrophilic layer. Russian Chemical Bulletin 53, 137–143 (2004). https://doi.org/10.1023/B:RUCB.0000024842.72905.c7
Issue Date:
DOI: https://doi.org/10.1023/B:RUCB.0000024842.72905.c7