Abstract
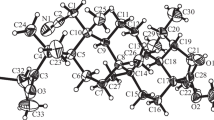
Starting with allobetulin triterpenoid A-nor-derivatives with a cis-junction of A/B rings were prepared for the first time.
Similar content being viewed by others
REFERENCES
Li, T.-S., Wang, J.-X., and Zheng, X.-J., J. Chem. Soc., Perkin, Trans. I, 1998, p. 3957.
Lavoie, S., Pichette, A., Garneau, F-X., Girard, M., and Gaudet, D., Synth. Commun., 2001, vol. 31, no. 1, p. 156.
Lugemwa, F.N., Huang, F.Y., Bentley, M.D., Mendel, M.J., and Alford, A.R., J. Agric. Food, Chem., 1990, vol. 38, p. 493.
Klinot, J., Budesinsky, M., and Svetly, J., Coll. Czech. Chem. Commun., 1990, vol. 55, p. 766.
Odinokova, L.E., Denisenko, M.V., Denisenko, V.A., and Uvarova, N.I., KhPS., 1988, p. 212.
Hase, T.A., Suokas, E., and Weckman, A., Synth. Commun., 1981, vol. 11, p. 489.
Berti, G., Bottari, F., Marsili, A., and Morelli, I., Tetrahedron, 1971, vol. 27, p. 2143.
Platonov, V.G., Zorina, A.D., Gordon, M.A., Chizhov, N.P., Balykina, L.V., Mikhailov, Yu.D., Ivanen, D.R., Tran, Kim, Kvi, and Shavva, A.G., Khim.-Farm. Zh., 1995, no. 2, p. 42.
Flekhter, O.B., Nigmatullina, L.R., Baltina, L.A., Karachurina, L.T., Galin, F.Z., Zarudii, F.S., Tolstikov, G.A., Boreko, E.I., Pavlova, N.I., Nikolaeva, S.N., and Savinova, O.V., Khim.-Farm. Zh., 2002, vol. 36, no. 9, p. 26.
White, A., Horsington, E.J., Nedjar, N., Peakman, T.M., and Curiale, J.A., Tetrahedron Lett., 1998, vol. 39, p. 3931.
Tolstikov, G.A., Goryaev. M.I., and Tolstikova, L.F., Zh. Obshch. Khim., 1965, vol. 35, p. 91.
Tolstikov, G.A., Alibaeva, Kh.M., and Potapov, V.M., Zh. Org. Khim., 1969, vol. 5, p. 1631.
Konoike, T., Takahashi, K., Kitaura, Y., and Kanda, Y., Tetrahedron, 1999, vol. 55, p. 14901.
Honda, T. and Gribble, G.W., J. Org. Chem., 1998, vol. 63, p. 4846.
Honda, T., Gribble, G.W., Suh, N., Finlay, H.J., Rounds, B., Bore, L., Favaloro, F.G., Wang, Y., and Sporn, M.B., J. Med. Chem., 2000, vol. 43, p. 1866.
Anjaneyulu, A.S.R., Rao, M.N., Sree, A., and Murty, V.S., Indian J. Chem. B., 1980, vol. 19, p. 735.
Deng, Y. and Snyder, J.K., J. Org. Chem., 2002, vol. 67, p. 2864.
Hase, T., Chem. Commun., 1972, p. 755.
Klinot, J., Rozen, J., Klinotova, E., and Vystrcil, A., Coll. Czech. Chem. Commun., 1987, vol. 52, p. 493.
Klinot, J., Liska, J., Forgagova, A., Budesinsky, M., Protiva, J., Hilgard, S., and Vystrcil, A., Coll. Czech. Chem. Commun., 1989, vol. 54, p. 413.
Pettit, G.R., Green, B., and Bowyer, W.J., J. Org. Chem., 1961, vol. 26, p. 2879.
Voser, W., White, D.E., Heusser, H., Jeger, O., and Ruzicka, L., Helv. Shim. Acta, 1952, vol. 35, p. 830.
Barton, D.H.R., Ives, D.A.J., and Thomas, B.R., J. Chem. Soc., 1954, p. 903.
Tolstikov, G.A., Goryaev, M.I., and Tolstikova, L.F., Zh. Obshch. Khim., 1964, vol. 34, p. 2815.
Biellmann, J.F. and Ourrison, G.P. Bull. Soc. Shim., 1962, p. 341.
Dutta, S.R. and Pradhan, B.P., Indian J. Chem. B, 1982, vol. 21, p. 575.
Garman, R. and Cowley, D., Austral. J. Chem., 1965, vol. 18, p. 213.
Klinot, I. and Vystriel, A., Coll. Czech. Chem. Commun., 1962, vol. 27, p. 377.
Alibaeva, Kh.A., Kim, Khya, Ok, Goryaev, M.I., and Iricmetov, M.P., Izv. Akad. Nauk, Kaz. SSR, Ser. Khim., 1975, vol. 25, no. 6, p. 39.
Tolstikov, G.A. and Goryaev, M.I., Zh. Org. Khim., 1966, vol. 2, p. 1718.
Tolstikov, G.A., Alibaeva, Kh.M., and Goryaev, M.I., Zh. Org. Khim., 1969, p. 1625.
Dehaen, W., Voets, M., and Bakulev, V.A., Advances in Nitrogen Heterocycles, 2000, vol. 4, p. 37.
Britton, T.C., Lobl, T.J., and Chidester, C.G., J. Org. Chem., 1984, vol. 49, p. 4773.
Morzherin, Y.Y., Glukhareva, T.V., Slepukhina, I.N., Mokrushin, V.S., Tkachev, A.V., and Bakulev, V.A., Mendeleev Commun., 2000, p. 19.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Medvedeva, N.I., Flekhter, O.B., Tretyakova, E.V. et al. Synthetic Transformations of Higher Terpenoids: XI. Synthesis of A-Nor-5bH-19b,28-epoxy-18a-olean-3-one Derivatives. Russian Journal of Organic Chemistry 40, 1092–1097 (2004). https://doi.org/10.1023/B:RUJO.0000045887.67489.44
Issue Date:
DOI: https://doi.org/10.1023/B:RUJO.0000045887.67489.44