Abstract
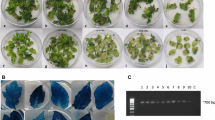
Tomato transformation and regeneration were analysed and optimized. Cotyledon explants from Lycopersicon esculentum cv. UC82B, were infected by Agrobacterium tumefaciens strain LBA4404 harbouring the neomycin phosphotransferase (NPTII) reporter gene. The effects of phenolic compounds, vitamins and growth regulators on plant transformation and regeneration were studied. Increasing the vitamin thiamine concentration from 0.1 mg l−1 in standard medium to 0.4 mg l−1 decreased the chlorophyll lost that accompanied the expansion of necrotic areas in cotyledon explants. Optimal shoot regeneration rate was obtained with a balanced concentration of 0.5 mg l−1 auxin indolelacetic acid (IAA) and 0.5 mg l−1 cytokinin zeatin riboside. Finally, when the phenolic acetosyringone was present in the co-culture medium at 200 µM, confirmed transgenic lines reached 50% of antibiotic resistant shoots. Under the above conditions, the transformation efficiency reached 12.5%.
Similar content being viewed by others
References
Beck MJ & Camper ND (1991) Shoot regeneration from petunia leaf disks as a function of explant size, configuration and benzyladenine exposure. Plant Cell Tiss. Org. Cult. 26: 101–106
Bevan M (1984) Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res. 12: 951–959
Bird CR, Smith CJS, Ray JA, Moureau P, Bevan MJ, Birds AS, Hughes S, Morris PC, Grierson D & Schuch W (1988) The tomato polygalacturonase gene and ripening specific expression in transgenic plants. Plant Mol. Biol. 11: 651–662
Bolton GW, Nester EW & Gordon MP (1986) Plant phenolic compounds induce expression of the Agrobacterium tumefaciens loci needed for virulence. Science 232: 983–985
Chyi YS & Phillips GC (1987) High efficiency Agrobacterium-mediated transformation of Lycopersicon based on conditions favourable for regeneration. Plant Cell Rep. 6: 105–108
Culianez-Macia FA & Hepburn AG (1988) The kinetics of T-strand production in a nopaline-type helper strain of Agrobacterium tumefaciens. Mol. Plant-Microbe Int. 5: 207–214
Davis MR & Miller LD (1991) Temporal competence for transformation of Lycopersicon esculentum (L. Mill) cotyledons by Agrobacterium tumefaciens: relation to wound healing and soluble plant factors. J. Exp. Bot. 42: 359–364
Ellul P, Garcia-Sogo B, Pineda B, Rios G, Roig LA & Moreno V (2003) The ploidy level of transgenic plants in Agrobacterium-mediated transformation of tomato cotyledons (Lycopersicon esculentum L. Mill.) is genotype and procedure dependent. Theor. Appl. Gen. 106(2): 231–238
Fillati JJ, Kiser J, Ronald R & Comai L (1987) Efficient transfer of glyphosate tolerance gene into tomato using a binary Agrobacterium tumefaciens vector. Biotechnology 5: 726–730
Garfinkel DJ & Nester EW (1980) Agrobacterium tumefaciens mutants affected in crown gall tumorigenesis and octopine catabolism. J. Bacteriol. 144: 732–743
Hamza S & Chupeau Y (1993) Re-evaluation of conditions for plant regeneration and Agrobacterium-mediated transformation from tomato (Lycopersicon esculentum). J. Exp. Bot. 269: 1837–1845
Hoekema A, Hirsch PR, Hooykaas PJJ & Schilperoort RA (1983) A binary plant vector strategy based on separation of vir-and T-region of the Agrobacterium tumefaciens Ti plasmid. Nature 303: 179–180
Janssens A, Genetello C, Van Montagu M & Zambrisky P (1986) Plant cells induce transcription of the Agrobacterium tumefaciens nopaline pTiC58 virulence region. Plant Sci. 47: 185–193
Koorneef M, Hanhart C, Jongsma M, Weide TIR, Zabel P & Hille J (1986) Breeding of a tomato genotype readily accessible to genetic manipulation. Plant Sci. 45: 201–208
Ling HQ, Kriseleit D & Ganal MW (1998) Effect of ticarcillin/potassium clavulanate on callus growth and shoot regeneration in Agrobacterium-mediated transformation of tomato (Lycopersicon esculentum Mill.) Plant Cell Rep. 17: 843–847
Linsmaier EM & Skoog F (1965) Organic growth factor requirements of tobacco tissue cultures. Physiol. Plant. 18: 100–127
McCormick S, Niedermeyer J, Fry J, Barnason A, Horsch R & Fraley R (1986) Leaf disc transformation of cultivated tomato (L. esculentum) using Agrobacterium tumefaciens. Plant Cell Rep. 5: 81–84
Murashige T & Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 15: 473–497
Ohki S, Bigot C & Mousseau J (1978) Analysis of shoot-formin capacity in vitro in two lines of tomato (Lycopersicon esculentum Mill) and their hybrids. Plant Cell Physiol. 19: 27–42
Pfitzner AJP (1998) Transformation of tomato. Meth. Mol. Biol. 81: 359–363
Shanin EA, Sukhapinda K & Simpson RB (1986) Transformation of cultivated tomato by a binary vector in Agrobacterium rhizogenes: transgenic plants with normal phenotypes harbour binary vector T-DNA, but no Ri-plasmid T-DNA. Theor. Appl. Gen. 72: 770–777
Sheikholeslam SN & Weeks DP (1987) Acetosyringone promotes high efficiency transformation of Arabidopsis thaliana explants by Agrobacterium tumefaciens. Plant Mol. Biol. 8: 291–298
Stachel SE, Nester EW & Zambryski PC (1986a) A plant cell factor induces Agrobacterium tumefaciens vir gene expression. Proc. Nat. Acad. Sci. USA 83: 379–383
Stachel SE, Nester EW & Zambryski PC (1986b) Generation of single-stranded T-DNA molecules during the initial stages of TDNA transfer from Agrobacterium tumefaciens to plant cells. Nature 322: 706–712
Ultzen T, Gielen J, Venema F, Westerbroek A, De Haan P, Tan M, Schram A, Van Grinsven M & Golbach R (1995) Resistance to tomato spotted wilt virus in transgenic tomato hybrids. Euphytica 85: 159–168
Yakuwa T, Harada T & Hanzawa M (1973) Basic studies on the vegetative propagation of horticultural plants. II. Effect of growth regulators on callus and organ formation from tissue segments of tomato cultured in vitro. Memories Faculty Agronomy Hokkaido University 9: 25–41
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cortina, C., Culiáñez-Macià, F.A. Tomato Transformation and Transgenic Plant Production. Plant Cell, Tissue and Organ Culture 76, 269–275 (2004). https://doi.org/10.1023/B:TICU.0000009249.14051.77
Issue Date:
DOI: https://doi.org/10.1023/B:TICU.0000009249.14051.77