Abstract
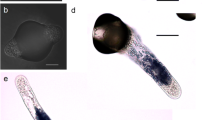
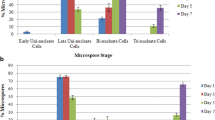
Anthers of Capsicum annuum L. were cultured on Murashige and Skoog (MS) medium containing 0.1 mg l−1 NAA and 0.1 mg l−1 kinetin. Inoculated anthers were subjected to 31 °C and development of microspores in anthers of varying stages was observed cytologically using 4′-6-diamidino-2-phenylindol-2HCl (DAPI). Pepper was characterized by a strong asynchrony of pollen development within a single anther. Percentage of pollen at different stages changed with the culture period, and the proportion of dead pollen increased drastically from day 2 after culture. Microspores that were cultured at the late-uninucleate stage followed one of two developmental pathways. In the more common route, the first sporophytic division was asymmetric and produced what appeared to be a typical bicellular pollen. Embryogenic pollen was formed by repeated divisions of the vegetative nucleus. In the second pathway, which occurred in fewer microspores, the first division was symmetric and both nuclei divided repeatedly to form embryogenic pollen. In early-bicellular pollen, sporophytic pollen was produced through division of the vegetative nucleus. In mid-bicellular pollen, the generative nucleus may undergo division to produce two or more sperm-like nuclei. However, division of the generative nucleus alone to form the embryo was never observed. The anther stage optimal for embryo production contained a large proportion (>75%) of early-binucleate pollen. Associations were found among the percentage of early-binucleate pollen, the frequency of embryogenic multinucleate pollen, and the yield of pollen embryos.
Similar content being viewed by others
References
Binarova P, Straatman K, Hause, B, Hause G & van Lammeren AAM (1993) Nuclear DNA synthesis during the induction of embryogenesis in cultured microspores and pollen of Brassica napus L. Theor. Appl. Genet. 87: 9–16
Binarova P, Hause G, Cenklova V, Cordewener JHG & Lookeren Campagne MM van (1997) A short severe heat shock is required to induce embryogenesis in late bicellular pollen of Brassica napus L. Sex. Plant Reprod. 10: 200–208
Bhojwani SS, Dunwell JM & Sunderland M (1973) Nucleic acid and protein contents of embryogenic tobacco pollen. J. Exp. Bot. 24: 863–871
Chen CC & Wu YH (1983) Segmentations in microspores of rice during anther culture. Proc. Natl. Sci. Counc. B. ROC 7: 151–157
Coleman AW & Goff LJ (1985) Applications of fluorochromes to pollen biology. 1. Mithramycin and 4',6-diamidino-2-phenylindole (DAPI) as vital stains and for quantitation of nuclear DNA. Stain Technol. 60: 145–154
Dunwell JM (1992) Mechanisms of microspore embryogenesis. In: Dattee Y, Dumas C & Gallais A (eds) Reproductive Biology and Plant Breeding (pp. 121–130). Springer, New York
Dunwell JM & Sunderland N (1974) Pollen ultrastructure in anther cultures of Nicotiana tabacum. II. Changes associated with embryogenesis. J. Exp. Bot. 25: 363–373
Duijs JG, Voorrips RE, Visser DL & Custers JBM (1992) Microspore culture is successful in most crop types of Brassica oleracea L. Euphytica 60: 45–55
Fan Z, Armstrong KC & Keller WA (1988) Development of microspores in vivo and in vitro in Brassica napus L. Protoplasma 147: 191–199
Gonzalez-Melendi P, Testillano PS, Ahmadian P, Fadon B, Vicente O & Risueno MC (1995) In situ characterization of the late vacuolate microspores as a convenient stage to induce embryogenesis in Capsicum. Protoplasma 187: 60–71
Guo Y, Sewon P & Pulli S (1999) Improved embryogenesis from anther culture and plant regeneration in timothy. Plant Cell Tiss. Org. Cult. 57: 85–93
Hause B & Hause G (1996) Induction of embryogenesis in isolated microspores and pollen of Brassica napus L. cv. Topas. Ph.D. Thesis, Wageningen Agricultural University
Hause B, Hause G, Pechan P & van Lammeren AAM (1993) Cytoskeletal changes in induction of embryogenesis in microspore and pollen cultures of Brassica napus L. Cell Biol. Int. 17: 153–168
Heberle-Bors E (1985) In vitro haploid formation from pollen: a critical review. Theor. Appl. Genet. 71: 361–374
Horner M & Street HE (1978) Pollen dimorphism-origin and significance in pollen plant formation by anther culture. Ann. Bot. 42: 763–771
Kim M (1999) The influence of temperature pretreatment on the production of microspore embryos in anther culture of Capsicum annuum L. Korean J. Plant Tiss. Cult. 26: 71–76
Kim M & Jang IC (2000) Rapid assessment of microspore development stage in pepper using DAPI and ferric chloride. J. Plant Biotech. 2: 129–134
Kim M & Jang IC (2001) Cytological analysis of microspores during temperature pretreatment in anther culture of Capsicum annuum L. Korean J. Plant Tiss. Cult. 28: 263–271
Kott LS, Polsoni L & Beversdorf WD (1988) Cytological aspects of isolated microspore culture of Brassica napus. Can. J. Bot. 66: 1658–1664
Murashige T & Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 15: 473–497
Nitsch C (1974) Pollen culture - a new technique for mass production of haploid and homozygous plants. In: Kasha KJ (ed) Haploid in Higher Plants - Advances and potential (pp. 123–134). University of Guelph, Guelph, Ont
Peng M, Ziauddin A & Wolyn DJ (1997) Development of asparagus microspores in vivo and in vitro is influenced by gametogenic stage and cold treatment. In Vitro Cell Dev. Biol. 33: 263–268
Pescitelli SM & Petolino JF (1988) Microspore development in cultured maize anthers. Plant Cell Rep. 7: 441–444
Raghavan V (1976) Role of generative cell in androgenesis in henbane. Science 191: 388–389
Raghavan V (1979) Embryogenic determination and ribonucleic acid synthesis in pollen grains of Hyoscyamus niger. Am. J.Bot. 66: 36–39
Regner F (1996) Anther and microspore culture in Capsicum. In: Jain SM, Sopory SK & Veilleux RE (eds) In Vitro Haploid Production in Higher Plants, Vol 3 (pp. 77–89). Kluwer Academic Publisher, Dordrecht
Reynolds TL (1986) Pollen embryogenesis in anther cultures of Solanum carolinense L. Plant Cell Rep. 5: 273–275
Reynolds TL (1993) A cytological analysis of microspores of Triticum aestivum (Poaceae) during normal ontogeny and induced embryogenic development. Am. J. Bot. 80: 569–576
Reynolds TL & Kitto SL (1992) Identification of embryoidabundant genes that are temporally expressed during pollen embryogenesis in wheat anther cultures. Plant Physiol. 100: 1744–1750
Rihova L & Tupy J (1999) Manipulation of division symmetry and developmental fate in cultures of potato microspores. Plant Cell Tiss. Org. Cult. 59: 135–145
Rose JB, Dunwell JM & Sunderland N (1986) Anther culture of Sorghum bicolor (L.)Moench II. Pollen development in vivo and in vitro. Plant Cell Tiss. Org. Cult. 6: 23–31
Rybczynski JJ, Simonson RL & Baenzicer PS (1991) Evidence for microspore embryogenesis in wheat anther culture. In Vitro Cell Dev. Biol. 20: 168–174
Sangwan RS & Sangwan-Norreel BS (1987) Ultrastructural cytology of plastids in pollen grains of certain androgenic and non-androgenic plants. Protoplasma 138: 11–22
Sunderland N & Wicks FM (1971) Embryoid formation in pollen grains of Nicotiana tabacum. J. Exp. Bot. 22: 213–226
Sunderland N, Roberts M, Evans LJ & Wildon DC (1979) Multicellular pollen formation in cultured barley anthers. J. Exp. Bot. 30: 1133–1144
Tanner GJ, Piccirilli M, Moore AE, Larkin PJ & Arcioni S (1990) Initiation of non-physiological division and manipulation of developmental pathway in cultured microspores of Medicago sp. Protoplasma 158: 165–175
Testillano PS, Gonzalez-Melendi P, Ahmadian P, Fadon B & Risueno MC (1995) The immunolocalization of nuclear antigens during the pollen developmental program and the induction of pollen embryogenesis. Exp. Cell Res. 221: 41–54
Touraev A, Pfosser M, Vicente O & Heberle-Bors E (1996) Stress as the major signal controlling the developmental fate of tobacco microspores: towards a unified model of induction of microspore/pollen embryogenesis. Planta 200: 144–152
Wilson HM, Mix G & Foroughi-Wher B (1978) Early microspore divisions and subsequent formation of microspore callus at high frequency in anthers of Hordeum vulgare. J. Exp. Bot. 29: 227–238
Zaki M & Dickinson HG (1991) Microspore-derived embryos in Brassica: the significance of division symmetry in pollen mitosis I to embryogenic development. Sex. Plant Reprod. 4: 48–55
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, M., Kim, J., Yoon, M. et al. Origin of Multicellular Pollen and Pollen Embryos in Cultured Anthers of Pepper (Capsicum Annuum). Plant Cell, Tissue and Organ Culture 77, 63–72 (2004). https://doi.org/10.1023/B:TICU.0000016506.02796.6a
Issue Date:
DOI: https://doi.org/10.1023/B:TICU.0000016506.02796.6a