Abstract
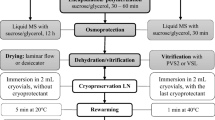
Embryogenic cell suspensions of two grapevine rootstocks: 110 Ritcher (V. berlandieri × V. rupestris), 41B (V. vinifera × V. berlandieri) and several table grape and wine cultivars (Vitis vinifera) were successfully cryopreserved by the encapsulation–vitrification method. Embryogenic cell suspensions were precultured for 3 days in liquid MGN medium supplemented with daily increasing sucrose concentrations of 0.25, 0.5, 0.75 M. Precultured cells were encapsulated and directly dehydrated with a highly concentrated vitrification solution prior to immersion in liquid nitrogen for 1 h. After rewarming at 40 °C for 3 min, cryopreserved cells were post-cultured on solid MGN medium supplemented with 2.5 g l−1 activated charcoal. Surviving cells were transferred to solid MGN medium for regrowth or solid MG medium for embryo development and then to solid WPM for plant regeneration. Optimal viability was 42–76% of cryopreserved cells when cell suspensions were precultured with a final sucrose concentration of 0.75 M and dehydrated with PVS2 at 0 °C for 270 min. Biochemical analysis showed that sucrose preculture caused changes in levels of total soluble protein and sugars in cell suspensions. Although the increase in fresh weight was significantly lower in cryopreserved cells than in control cells, the growth pattern of the cryopreserved cells and control cells was the same after two subcultures, following re-establishment in cell suspensions. Protocol developed in this study suggests a universal and highly efficient cryopreservation system suitable for several genetically diversed Vitis species.
Similar content being viewed by others
References
Bachiri Y, Gazeau C, Hansz J, Morisset C & Dereuddre (1995) Successful crypreservation of suspension cells by encapsulation- dehydration. Plant Cell Tiss. Org. Cult. 43: 241–248
Bornhoff BA & Harst M (2000) Establishment of embryo suspension cultures of grapevines (Vitis L.). Vitis 39: 27–29
Bradford MM (1976) A rapid and sensitive method for the quanti-fication of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 72: 248–254
Coutos-Thevenot P, Goebel-Trourand I, Mauro MC, Jouanneau JP, Boulay M, Deloire A & Guern J (1992) Somatic embryogenesis from grapevine cells. I. Improvement of embryo development by changes in culture conditions. Plant Cell Tiss. Org. Cult. 29: 125–133
Crowe JH, Crowe LM, Carpenter JF & Wistrom CA (1987) Stabilization of dry phospholipid bilayers and proteins by sugars. Biochem. J. 242: 1–10
Dussert S, Mauro MC, Deloire A, Hamon A & Engelmann F (1991) Cryopreservation of grape embryogenic cell suspensions. 1. Influence of pretreatment, freezing and thawing conditions. Cryo-Lett. 12: 287–298
Dussert S, Mauro MC & Engelmann F (1992) Cryopreservation of grape embryogenic cell suspensions. 2. Influence of post-culture conditions and application to different strains. Cryo-Lett. 13: 15–22
Engelmann F (1997) In vitro conservation methods. In: Callow JA, Ford-Lloyd BV & Newbury HJ (eds) Biotechnology and Plant Genetic Resources (pp. 119–161). CAB International, Oxford
Hirai D, Shirai K, Shirai S & Sakai A (1998) Cryopreservation of in vitro-grown meristems of strawberry (Fragaria × ananassa Duch.) by encapsulation-vitrification. Euphytica 101: 109–115
Huang Ch-N, Wang J-H, Yan Q-Sh, Zhang X-Q & Yan Q-F (1995) Plant regeneration from rice (Oryza sativa L.) embryogenic suspension cells cryopreserved by vitrification. Plant Cell Rep. 14: 730–734
Ishikawa M, Tandon P, Suzuki M & Yamaguishi-Ciampi A (1996) Cryopreservation of bromegrass (Bromus inermis Leyss) suspension cultured cells using slow prefreezing and vitrification procedures. Plant Sci. 120: 81–88
Jayasankar S, Van Aman M, Li Zh & Gray DJ (2001) Direct seedling of grapevine somatic embryos and regeneration of plants. In Vitro Cell Dev. Biol-Plant 37: 476–479
Jitsuyama Y, Suzuki T, Harada T & Fujikawa S (2002) Sucrose incubation increases freezing tolerance of Asparagus (Asparagus officinalis L.) embryogenic cell suspensions. Cryo-Lett. 23: 103–112
Kalengamaliro NE, Gana JA, Cunningham SM & Volenec JJ (2000) Mechanisms regulating differential freezing tolerance of suspension cell cultures derived from contrasting alfalfa genotypes. Plant Cell Tiss. Org. Cult. 61: 143–151
Kikkert JR, Thomas MR & Reisch BI (2001) Grapevine genetic engineering. In: Roubelakis-Angelakis KA (ed) Molecular Biology and Biotechnology of the Grapevine (pp. 393–410). Kluwer Academic Publishers, Dordrecht
Leborgne N, Teulieres Ch, Travert S, Rols M-P, Teissie J & Boudet AM (1995) Introduction of specific carbohydrates into Eucalyptus gunnii cells increases their freezing tolerance. Eur. J. Biochem. 229: 710–717
Lloyd G & McCown B (1980) Commercially feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Proc. Intl. Plant Prop. Soc. 30: 421–427
Martinelli L & Gribaudo I (2001) Somatic embryogenesis in grapevine. In: Roubelakis-Angelakis KA (ed) Molecular Biology and Biotechnology of the Grapevine (pp. 327–351). Kluwer Academic Publishers, Dordrecht
Mauro MC, Toutain S, Walter B, Pinck L, Otten L, Coutos-Thevenot P, Deloire A & Barbier P (1995) High efficiency regeneration of grapevine plants transformed with the GFLV coat protein gene. Plant Sci. 112: 97–106
Mullins MG & Srinivasan C (1976) Somatic embryos and plantlets from an ancient clone of the grapevine (cv. Cabernet-Sauvignon) by apomixis in vitro. J. Exp. Bot. 27: 1022–1030
Niino T, Tashiro K, Suzuki M, Ohuchi S, Magoshi J & Akihama T (1997) Cryopreservation of in vitro grown shoot tips of cherry and sweet cherry by one-step vitrification. Sci. Hort. 70: 155–163
Nishizawa S, Sakai A, Amano Y & Matsuzawa T (1993) Cryopreservation of asparagus (Asparagus officinalis L.) embryogenic suspension cells and subsequent plant regeneration by vitrification. Plant Sci. 91: 67–73
Nitsch JP & Nitsch C (1969) Haploid plants from pollen grains. Science 163: 85–87
Perl A, Saad S, Sahar N & Holland D (1995) Establishment of longterm embryogenic cultures of seedless Vitis vinifera cultivar - a 275synergistic effect of auxins and the role of abscisic acid. Plant Sci. 104: 193–200
Rall WF (1987) Factors affecting the survival of mouse embryos cryopreserved by vitrification. Cryobiology 24: 367–402
Sakai A, Kobayashi S & Oiyama I (1990) Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tanaka) by vitrification. Plant Cell Rep. 9: 30–33
Sakai A, Kobayashi S & Oiyama I (1991) Survival by vitrification of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tanaka) cooled to-196 ?C. J. Plant Physiol. 137: 465–470
Shibli RA, Haagenson DM, Cunningham SM, Berg WK & Volenec JJ (2001) Cryopreservation of alfalfa (Medicago sativa L.) cells by encapsulation-dehydration. Plant Cell Rep. 20: 445–450
Steponkus PL & Lanphear FO (1967) Refinement of the triphenyltetrazolium chloride method of determining cold injury. Plant Physiol.42: 1423–1426
Vandenbussche B, Leuridan S, Verdoodt V, Gysemberg M & De Proft M (1999) Changes in sugar content and fatty acid composition of in vitro sugar beet shoots after cold acclimation: influence on survival after cryopreservation. Plant Growth Reg. 28: 157–163
Wang QC, Gafny R, Sahar N, Sela I, Mawassi M, Tanne E & Perl A (2002) Cryopreservation of grapevine (Vitis vinifera L.) embryogenic cell suspensions and subsequent plant regeneration by encapsulation-dehydration. Plant Sci. 162: 551–558
Zamski E & Grunberger Y (1995) Short-and long-eared highyielding hexaploid wheat cultivars: which has unexpressed potential for higher yield? Ann. Bot. 75: 501–506
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Q., Mawassi, M., Sahar, N. et al. Cryopreservation of Grapevine (Vitis spp.) Embryogenic Cell Suspensions by Encapsulation–Vitrification. Plant Cell, Tissue and Organ Culture 77, 267–275 (2004). https://doi.org/10.1023/B:TICU.0000018393.58928.b1
Issue Date:
DOI: https://doi.org/10.1023/B:TICU.0000018393.58928.b1