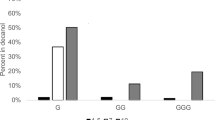
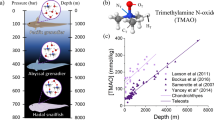
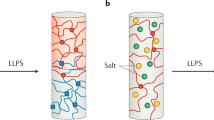
Abstract
IN 1959, Lovelock and Bishop1 reported the protective action of dimethylsulphoxide (DMSO) in preventing freezing injury to living calls. The “cryoprotective” action of this substance, now in wide use2 for the frozen preservation of various types of living cell, presumably resides in part in its ability to reduce the extent to which salts are concentrated when water is converted to ice. On the basis of this assumption Farrant3,4 proposed and developed two procedures by which DMSO might be used to prevent altogether the concentration of the soluble electrolytes in tissues cooled to temperatures in the range −78° to −196° C. In the course of his studies, Farrant obtained preliminary data describing the phase equilibrium behaviour of the system water–DMSO, both in the absence and in the presence of small quantities of salts. In view of the current interest in the use of DMSO we undertook a more extensive determination of the phase equilibrium and non-equilibrium behaviour of solutions of this compound in water. These measurements form the subject of this communication. They indicate (1) that the system water–DMSO can, in all proportions, be crystallized completely, and (2) that, in certain conditions, a stable hydrate (DMSO.3H2O) is formed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Lovelock, J. E., and Bishop, M. W. H., Nature, 183, 1394 (1959).
Meryman, H. T., in Cryobiology (edit. by Meryman, H. T.), (Academic Press, London and New York, 1966).
Farrant, J., Nature, 205, 1284 (1965).
Farrant, J., Walter, C. A., and Armstrong, J. A., Proc. Roy. Soc., B, 168, 293 (1967).
Asahina, E., Fed. Proc., 24, S-183 (1965).
Leibo, S. P., and Mazur, P., Cryobiology, 3, 366 (Abstract, 1967).
Leibo, S. P., and Mazur, P., Cryobiology, 4, 252 (Abstract, 1968).
Greaves, R. I. N., and Davies, J. D., Ann. NY Acad. Sci., 125, 548 (1965).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
RASMUSSEN, D., MACKENZIE, A. Phase Diagram for the System Water–Dimethylsulphoxide. Nature 220, 1315–1317 (1968). https://doi.org/10.1038/2201315a0
Received:
Issue Date:
DOI: https://doi.org/10.1038/2201315a0
This article is cited by
-
Solvatochromism, Preferential Solvation and Multiparametric Approach to the Spectral Shift of Methyl Orange in Aqueous Cosolvent Mixtures
Journal of Fluorescence (2024)
-
Functionalized Hydrogel-Based Wearable Gas and Humidity Sensors
Nano-Micro Letters (2023)
-
A standard operating procedure for an enzymatic activity inhibition assay
European Biophysics Journal (2021)
-
Molecular dynamics simulations of a DMSO/water mixture using the AMBER force field
Journal of Molecular Modeling (2018)
-
Low-dose action of tryptanthrin and its derivatives against developing embryos of the sea urchin Strongylocentrotus intermedius
Environmental Monitoring and Assessment (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.