Abstract
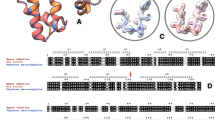
MOST enzymes are quickly inactivated above about 55 °C but those from thermophile bacteria are stable for long periods at higher temperatures1. We do not know why because so far their structures have proved too complex. For example although the tertiary and quaternary structures of the enzyme glyceraldehyde phosphate dehydrogenase from lobster muscle and from Bacterium stearothermophilus are alike their amino acid sequences differ by more than 130 out of some 330 positions which makes it hard to decide why the stearothermophilus enzyme is more stable. The electron transfer protein ferredoxin offers a better chance because its single polypeptide chain contains fewer than 60 residues; its structure is known and its heat stability and amino acid sequence have been determined in both mesophile and thermophile bacteria. We have built an atomic model of this protein, replaced its amino acid side chains in turn to correspond to the published sequences and searched for possible causes of the greater heat stability of ferredoxins from thermophile bacteria. We found that it arises mainly from external salt bridges linking residues near the amino terminus to others near the carboxy terminus. Haemoglobin A2 a minor fraction of adult human haemoglobin which is a little more heat stable than the major fraction, haemoglobin A, seemed another good choice because its amino acid sequence differs from that of A at only 10 positions. The atomic model suggests that at only two of these positions are the replacements likely to contribute to the extra stability of haemoglobin A2 one replacement providing an extra hydrogen bond between the α1 and β1 subunits and the other adding two non-polar interactions to a surface crevice within the β subunits. To account for the increased heat stability of the two proteins the extra bond energy provided by these interactions need not be larger than 10 kJ for ferredoxin or 5 kJ for haemoglobin A2.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Hocking, J. D., and Harris, J. I., FEBS Lett., 34, 280–284 (1973).
Adman, E. T., Sieker, L. C., and Jensen, L. H., J. biol. Chem., 248, 3987–3996 (1973).
Devanathan, T., Akagi, J. M., Hersh, R. T., and Himes, R. H., J. biol. Chem., 244, 2846–2853 (1969).
Kinderlehrer, J., Lehmann, H., and Tipton, K. F., Biochem. J., 135, 805–815 (1973).
Perutz, M. F., Muirhead, H., Cox, J. M., and Goaman, L. C. G., Nature, 219, 131–139 (1968).
Perutz, M. F., Nature, 247, 341–344 (1974).
Tanaka, M., Nakashima, T., Benson, A. M., Mower, H. F., and Yasunobu, K. T., Biochemistry, 5, 1666–1681 (1966).
Rall, S. C., Bolinger, R. E., and Cole, R. D., Biochemistry, 8, 2486–2496 (1969).
Tsunoda, J. N., Yasunobo, K. T., and Whiteley, H. R., J. biol. Chem., 243, 6262–6272 (1968).
Tanaka, M., et al., J. biol. Chem., 246, 3953–3960 (1971).
Tanaka, M., Haniu, M., Yasunobu, K. T., Himes, R. H., and Akagi, J. M., J. biol. Chem., 248, 5215–5217 (1973).
Dayhoff, M. O., Atlas of Protein Sequence and Structure (National Biomedical Research Foundation, Washington DC, 1972).
Yasunobu, K. T., and Tanaka, M., in Iron-Sulfur Proteins, 2 (edit. by Lovenberg), 27 (Academic, London and New York, 1973).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
PERUTZ, M., RAIDT, H. Stereochemical basis of heat stability in bacterial ferredoxins and in haemoglobin A2. Nature 255, 256–259 (1975). https://doi.org/10.1038/255256a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/255256a0
This article is cited by
-
Acetylation regulates the oligomerization state and activity of RNase J, the Helicobacter pylori major ribonuclease
Nature Communications (2023)
-
Intrinsic basis of thermostability of prolyl oligopeptidase from Pyrococcus furiosus
Scientific Reports (2021)
-
A similarity distance of diversity measure for discriminating mesophilic and thermophilic proteins
Amino Acids (2013)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.