Abstract
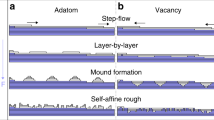
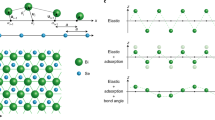
Models of crystal growth have been defined by comparing macroscopic growth kinetics with theoretical predictions for various growth mechanisms1,2. The classic Burton–Cabrera–Frank (BCF) theory3 predicts that spiral growth at screw dislocations will dominate near equilibrium. Although this has often been observed2,4, such growth is sometimes inhibited4,5, which has been assumed to be due to the presence of impurities6. At higher supersaturations, growth is commonly modelled by two-dimensional nucleation on the pre-existing surface according to the ‘birth and spread’ model7. In general, the morphology of a growing crystal is determined by the rate of growth of different crystallographic faces, and periodic-bond-chain (PBC) theory8,9 relates this morphology to the existence of chains of strongly bonded ions in the structure. Here we report tests of such models for the growth of barite crystals, using a combination of in situ observations of growth mechanisms at molecular resolution with the atomic force microscope10,11 and computer simulations of the surface attachment of growth units. We observe strongly anisotropic growth of two-dimensional nuclei with morphologies controlled by the underlying crystal structure, as well as structure-induced self-inhibition of spiral growth. Our results reveal the limitations of both the BCF and PBC theories in providing a general description of crystal growth.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Chernov, A. A. Modern Crystallography III (Crystal Growth) (Springer, Berlin, 1984).
Sunagawa, I. in Morphology of Crystals (ed. Sunagawa, I.) 323–365 (Terra Scientific, Tokyo, 1987).
Burton, W. K., Cabrera, N. & Frank, F. C. The growth of crystals and the equilibrium structures of their surfaces. Phil. Trans. R. Soc. 243, 299–358 (1951).
Bennema, P. Spiral growth and surface roughening: developments since Burton, Cabrera and Frank. J.Cryst. Growth 69, 182–197 (1984).
Bennema, P. & Gilmer, J. in Crystal Growth: An Introduction (ed. Hartman, P.) 263–327 (North Holland, Amsterdam, 1973).
Botsaris, G. D. in Industrial Crystallization 81 (eds Jancic, S. J. & de Jong, E. J.) (North Holland, Amsterdam, 1982).
Nielsen, A. E. Kinetics of Precipitation (Pergamon, Oxford, 1964).
Hartman, P. in Morphology of Crystals (ed. Sunagawa, I.) 269–319 (Terra Scientific, Tokyo, 1987).
Hartman, P. & Strom, C. S. Structural morphology of crystals with the barite (BaSO4) structure: a revision and extension. J. Cryst. Growth 97, 502–512 (1989).
Hillner, P. E., Gratz, A. J., Manne, S. & Hansma, P. K. Atomic-scale imaging of calcite growth and dissolution in real time. Geology 20, 359–362 (1992).
Dove, P. M. & Chermak, J. in CMS Workshop Lectures: Scanning Probe Microscopy of Clay Minerals (eds Nagy, K. L. & Blum, A. E.) 139–170 (Clay Minerals Soc., Washington DC, 1994).
Allan, N. L. et al. Calculated bulk and surface properties of sulfates. Faraday Disc. 95, 273–280 (1993).
Miyaka, M., Minato, I., Morikawa, H. & Iwai, S. Crystal structures and sulphate force constants of barite, celestite and anglesite. Am. Mineral. 63, 506–510 (1978).
Hartman, P. & Perdok, W. G. On the relations between structure and morphology of crystals. Acta Crystallogr. 8, 48–52 (1955).
Hottenhuis, M. H. J. & Lucasius, C. B. The influence of internal crystal structure on surface morphology; in situ observations of potassium hydrogen phthalate {010}. J. Cryst. Growth 94, 708–720 (1989).
Hartman, P. & Heijnen, W. M. M. Growth mechanism of a crystal face for which more than one surface structure is possible. J. Cryst. Growth 63, 261–264 (1983).
Conway, B. E. Ionic Hydration in Chemistry and Biophysics (Elsevier Scientific, Amsterdam, 1980).
Rashin, A. A. & Honig, B. Reevaluation of the Born model of ion hydration. J. Phys. Chem. 89, 5588–5593 (1985).
Becker, U., Pina, C. M., Risthaus, P., Bosbach, D. & Putnis, A. Experimental and theoretical treatment of molecular-scale mechanisms of crystal growth on barite (Eighth annual V. M. Goldschmidt Conference, Toulouse, France, 1998).
Acknowledgements
We thank the Deutsche Forschungsgemeinschaft (DFG) and the Spanish Ministry of Science and Culture for financial support for this project, and A. Pina for Fig. 3a.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pina, C., Becker, U., Risthaus, P. et al. Molecular-scale mechanisms of crystal growth in barite. Nature 395, 483–486 (1998). https://doi.org/10.1038/26718
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/26718
This article is cited by
-
How ice grows from premelting films and water droplets
Nature Communications (2021)
-
Intermobility of barium, strontium, and lead in chloride and sulfate leach solutions
Geochemical Transactions (2019)
-
Deciphering pore-level precipitation mechanisms
Scientific Reports (2017)
-
A multistep attachment process: Transformation of titanate nanotubes into nanoribbons
Science China Chemistry (2012)
-
The growth of the screw dislocation of nacreous layer on Pteria penguin
Science China Earth Sciences (2011)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.