Abstract
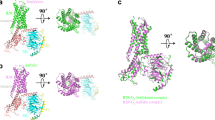
Inhibitors of the conversion of angiotensinogen to the vasoconstrictor angiotensin II have considerable value as antihypertensive agents. For example, captopril and enalapril are clinically useful as inhibitors of angiotensin-converting enzyme. This has encouraged intense activity in the development of inhibitors of kidney renin, which is a very specific aspartic proteinase catalysing the first and rate limiting step in the conversion of angiotensinogen to angiotensin II. The most effective inhibitors such as H-142 (ref. 1) and L-363,564 (ref. 2) have used non-hydrolysable analogues of the proposed transition state3–6, and partial sequences of angiotensinogen (Table I)7–9. H-142 is effective in lowering blood pressure in humans10,11but has no significant effect on other aspartic proteinases such as pepsin in the human body (Table 1). At present there are no crystal structures available for human or mouse renins12 although three-dimensional models13–16demonstrate close structural similarity to other spartic proteinases. We have therefore determined by X-ray analysis the three-dimensional structures of H-142 and L-363,564 complexed with the aspartic proteinase endothiapepsin17–19, which binds these inhibitors with affinities not greatly different from those measured against human renin (Table 1). The structures of these complexes and of that between endothiapepsin and the general aspartic proteinase inhibitor, H-256 (Table 1) define the common hydrogen bonding schemes that allow subtle differences in side-chain orientations and in the positions of the transition state analogues with respect to the active-site aspartates.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
1. Szelke, M. et al. Nature 299, 555–557 (1982). 2. Boger, J. et al. Nature 303, 81–84 (1983). 3. Hallett, A. et al. in Aspartic Proteinases and their Inhibitors (ed. Kostka, V.) 467–478 (de Gruyter, Berlin, 1985). 4. Dunn, B. M., Kammermann, B. & McCurry, K. Analyt. Biochem. 138, 68–73 (1984). 5. Szelke, M., Jones, D. M., Atrash, B., Hallett, A. & Leckie, B. J. in Peptides, Structure and Function: Proceedings of the Eighth American Peptide Symposium (eds Hruby, V. J. & Rich, D. H.) 579–582 (Pierce Chemical, Rockford, Illinois, 1983). 6. Szelke, M. in Aspartic Proteinases and their Inhibitors (ed. Kostka, V.) 421–441 (de Gruyter, Berlin, 1985). 7. Skeggs, L. T., Lentz, K., Hochstrasser, H. & Kahn, J. R. Can. med. Ass. J. 90,185–189 (1964). 8. Kokusbu, T. et al. Nature 217, 456–457 (1968). 9. Haber, E. & Burton, J. Fedn Proc. 38, 2768–2773 (1979). 10. Webb, D. J. et al. Lancet ii, 1486–1487 (1983). 11. Webb, D. J. et al. J. Hypertension 3, 653–658 (1985). 12. Navia, M. A., Springer, J. P., Poe, M., Boger, J. & Hoogsteen, K. / biol. Chem. 259, 12714–12717 (1984). 13. Blundell, T. L., Sibanda, B. L. & Pearl, L. H. Nature 304, 273–275 (1983). 14. Sibanda, B. L. et al. FEES Lett. 174, 102–111 (1984). 15. Akahane, K. et al. Hypertension 7, 3–12 (1985). 16. Carlson, W., Karplus, M. & Haber, E. Hypertension 7, 13–19 (1985). 17. Pearl, L. H. & Blundell, T. L. FEBS Lett. 174, 96–101 (1984). 18. Pedersen, V. B. Biochemistry (in the press). 19. Blundell, T. L., Jenkins, J., Pearl, L. H., Sewell, T. & Pedersen, V. in Aspartic Proteinases and their Inhibitors (ed. Kostka, V.) 151–161 (de Gruyter, Berlin, 1985). 20. James, M. N. G. & Sielecki, A. /. molec. Biol. 163, 299–361 (1983). 21. Tang, J., James, M. N. G., Hsu, I. N., Jenkins, J. A. & Blundell, T. L. Nature 271, 618–621 (1978). 22. Bott, R., Subramanian, E. & Davies, D. R. Biochemistry 21, 6956–6962 (1982). 23. James, M. N. G., Sielecki, A. R., Salituro, R, Rich, D. H.. & Hofmann, T. Proc. natn. Acad. Sc. U.S.A. 79, 6137–6141 (1982). 24. James, M. N. G. & Sielecki, A. R. Nature 319, 33–39 (1986). 25. Boger, J. in Aspartic Proteinases and their Inhibitors (ed. Kostka, V.) 401–420 (de Gruyter, Berlin, 1985). 26. Dunn, B. M. et al. in Aspartic Proteinases and their Inhibitors (ed. Kostka, V.) 221–243 (de Gruyter, Berlin, 1985). 27. Jones, T. A. Appl. crystallogr. 11, 268–272 (1978). 28. Haneef, I., Moss, D. S., Stanford, M. J. & Borkakoti, N. Acta crystallogr. A41,426–433 (1985).
Author information
Authors and Affiliations
Author notes
To whom correspondence should be addressed.
- T. L. Blundell
Rights and permissions
About this article
Cite this article
Foundling, S., Cooper, J., Watson, F. et al. High resolution X-ray analyses of renin inhibitor-aspartic proteinase complexes. Nature 327, 349–352 (1987). https://doi.org/10.1038/327349a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/327349a0
This article is cited by
-
Computer simulation of the conformational behaviour of angiotensinogen (6?13) renin substrate
Journal of Computer-Aided Molecular Design (1992)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.