Abstract
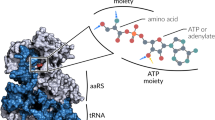
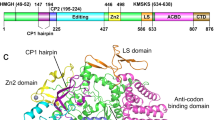
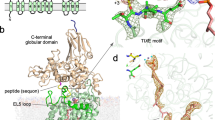
The three-dimensional crystal structure of seryl-transfer RNA synthetase from Escherichia coli, refined at 2.5 Å resolution, is described. It has an N-terminal domain that forms an antiparallel α helical coiled-coil, stretching 60 Å out into the solvent and stabilized by interhelical hydrophobic interactions and an active-site α – β domain based around a seven-stranded antiparallel β sheet. Unlike the three other known synthetase structures, the enzyme contains no classical nucleotide-binding fold, and is the first representative of a second class of aminoacyl-tRNA synthetase structures.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Schimmel, P. A. Rev. Biochem. 56, 125–158 (1987).
Crick, F. H. C. Symp. Soc. exp. Biol. 12, 138–163 (1958).
Brick, P. & Blow, D. M. J. molec. Biol. 194, 287–297 (1987).
Brick, P., Bhat, T. N. & Blow, D. M. J. molec. Biol. 208, 83–98 (1989).
Brunie et al. J. molec. Graph. 5, 18–21 (1987).
Rossmann, M. G., Moras, D. & Olsen, K. W. Nature 250, 194–199 (1974).
Blow, D. M. et al. J. molec. Biol. 171, 571–576 (1983).
Webster, T. A., Lathrop, R. H. & Smith, T. F. Biochemistry 26, 6950–6957 (1987).
Burbaum, J. J., Starzyk, R. M. & Schimmel, P. Proteins 7, 99–111 (1990).
Rould, M. A., Perona, J. J., Söll, D. & Steitz, T. A. Science 248, 1135–1142 (1989).
Grosjean, H., Nicoghosian, K., Haumont, E., Söll, D. & Cedergren, R. Nucleic Acids Res. 13, 5697–5706 (1985).
Leinfelder, W., Zehelein, E., Mandrand-Berthelot, M-A. & Böck, A. Nature 331, 723–725 (1988).
Normanly, J., Ogden, R. C., Horvayh, S. J. & Abelson, J. Nature 321, 213–219 (1986).
Schulman, L. H. & Pelka, H. Nucleic Acids Res. 18, 285–289 (1990).
Härtlein, M., Madern, D. & Leberman, R. Nucleic Acids Res. 15, 1005–1017.
Leberman, R., Berthet-Colominas, C., Cusack, S. & Härtlein, M. J. molec. Biol. 193, 423–425 (1987).
Kabsch, W. & Sander, C. Biopolymers 22, 2577–2637 (1983).
Cohen, C. & Parry, D. A. D. Proteins 7, 1–15 (1990).
Richardson, J. S. & Richardson, D. C. in Prediction of Protein Structure and the Principles of Protein Conformation (ed. Fasman, G. D.) 1–98 (Plenum New York, 1989).
Roth, M. et al. Nature 340, 659–662 (1989).
Hountondji, C., Dessen, P. & Blanquet, S. Biochimie 68, 1071–1078 (1986).
Jacobo-Molina, A., Peterson, R. & Yang, D. C. H. J. biol. Chem. 264, 16608–16612 (1989).
Anselme, J. & Härtlein, M. Gene 84, 481–485 (1989).
Léveque, F., Plateau, P., Dessen, P. & Blanquet, S. Nucleic Acids Res. 18, 305–311 (1990).
Eriani, G. et al. Nature 347, 203–206 (1990).
Ruff, M. thesis, Univ. Louis Pasteur, Strasburg (1990).
Wetzel, R. Origins of Life 9, 39–50 (1978).
Härtlein, M. & Madern, D. Nucleic Acids Res. 15, 10199–10210 (1987).
Landschulz, W. H., Johnson, P. F. & McKnight, S. L. Science 240, 1759–1764 (1988).
Vinson, C. R., Sigler, P. B. & McKnight, S. L. Science 246, 911–916 (1989).
O'Shea, E. K., Rutkowski, R. & Kim, P. S. Science 243, 538–542 (1989).
Oas, T. G., McIntosh, L. P., O'Shea, E. K., Dahlquist, F. W. & Kim, P. S. Biochemistry 29, 2891–2894 (1990).
Banner, D. W., Kokkinidis, M. & Tsernoglou, D. J. molec. Biol. 196, 657–675 (1987).
Helmer-Citterich, M., Anceschi, M. M., Banner, D. W. & Cesareni, G. EMBO J. 7, 557–566 (1988).
Moine, H. et al. Proc. natn. Acad. Sci. U.S.A. 85, 7892–7896 (1988).
Putzer, H., Brakhage, A. A. & Grunberg-Manago, M. J. Bact. 172, 4593–4602 (1990).
Leslie, A. Acta crystallogr. 43, 134–137 (1974).
Jones, T. A. & Thirup, S. EMBO J. 5, 819–822 (1986).
Fujinaga, M., Gros, P. & van Gunsteren, W. F. J. appl. Crystallogr. 22, 1–8 (1989).
Brunie, S., Zelwer, C. & Risler, J-L. J. molec. Biol. (in the press).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cusack, S., Berthet-Colominas, C., Härtlein, M. et al. A second class of synthetase structure revealed by X-ray analysis of Escherichia coli seryl-tRNA synthetase at 2.5 Å. Nature 347, 249–255 (1990). https://doi.org/10.1038/347249a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/347249a0
This article is cited by
-
Symmetrical distributions of aminoacyl-tRNA synthetases during the evolution of the genetic code
Theory in Biosciences (2023)
-
The structural basis of the genetic code: amino acid recognition by aminoacyl-tRNA synthetases
Scientific Reports (2020)
-
Aminoacyl tRNA synthetases as malarial drug targets: a comparative bioinformatics study
Malaria Journal (2019)
-
Clone and functional analysis of Seryl-tRNA synthetase and Tyrosyl-tRNA synthetase from silkworm, Bombyx mori
Scientific Reports (2017)
-
Neurodegenerative disease-associated mutants of a human mitochondrial aminoacyl-tRNA synthetase present individual molecular signatures
Scientific Reports (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.