Abstract
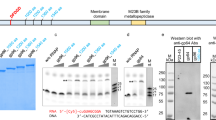
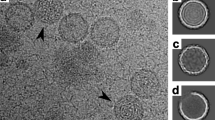
DOUBLE-STRANDED RNA viruses have an RNA-dependent RNA polymerase activity associated with the viral particles which is indispensable for their replication cycle. Using the yeast L-A double-stranded RNA virus we have investigated the mechanism by which the virus encapsidates its genomic RNA and RNA polymerase. The L-A gag gene encodes the principal viral coat protein and the overlapping pol gene is expressed as a gag–pol fusion protein which is formed by a −1 ribosomal frameshift1–3. Here we show that Gag alone is sufficient for virus particle formation, but that it fails to package the viral single-stranded RNA genome. Encapsidation of the viral RNA requires only a part of the Pol region (the N-terminal quarter), which is presumably distinct from the RNA polymerase domain. Given that the Pol region has single-stranded RNA-binding activity, these results are consistent with our L-A virus encapsidation model1: the Pol region of the fusion protein binds specifically to the viral genome (+) strand, and the N-terminal gag-encoded region primes polymerization of Gag to form the capsid, thus ensuring the packaging of both the viral genome and the RNA polymerase.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Fujimura, T. & Wickner, R. B. Cell 55, 663–671 (1988).
Icho, T. & Wickner, R. B. J. biol. Chem. 264, 6716–6723 (1989).
Dinman, J. D., Icho, T. & Wickner, R. B. Proc. natn. Acad. Sci. U.S.A. 88, 174–178 (1991).
Wickner, R. B. in The Molecular Biology of the Yeast Saccharomyces Vol. 1 (eds Broach, J. R., Jones, E. W. & Pringle, J. R.) 263–296 (Cold Spring Harbor Laboratory Press, New York, 1991).
Jacks, T., Madhani, H. D., Masiarz, F. R. & Varmus, H. E. Cell 55, 447–458 (1988).
Herring, A. J. & Bevan, E. A. Nature 268, 464–466 (1977).
Welsh, D. J. & Leibowitz, M. J. Nucleic Acids Res. 8, 2365–2375 (1980).
Hopper, J. E., Bostian, K. A., Rowe, L. B. & Tipper, D. J. J. biol. Chem. 252, 9010–9017 (1977).
Fujimura, T., Esteban, R. & Wickner, R. B. Proc. natn. Acad. Sci. U.S.A. 83, 4433–4437 (1986).
Fujimura, T. & Wickner, R. B. Molec. cell. Biol. 7, 420–426 (1987).
Esteban, R., Fujimura, T. & Wickner, R. B. EMBO J. 8, 947–954 (1989).
Fujimura, T., Esteban, R., Esteban, L. M. & Wickner, R. B. Cell 62, 819–828 (1990).
Wickner, R. B., Icho, T., Fujimura, T. & Widner, W. R. J. Virol. 65, 155–161 (1991).
Oertle, S. & Spahr, P. J. Virol. 64, 5757–5763 (1990).
Birnbaum, F. & Nassal, M. J. Virol. 64, 3319–3330 (1990).
Suzuki, M. EMBO J. 8, 797–804 (1989).
Churchill, M. E. A. & Travers, A. A. Trends Biochem. Sci. 16, 92–97 (1991).
Kamer, G. & Argos, P. Nucleic Acids Res. 12, 7269–7282 (1984).
Hirsch, R. C., Lavine, J. E., Chang, L.-J., Varmus, H. E. & Ganem, D. Nature 344, 552–555 (1990).
Bartenschlager, R., Junker-Niepmann, M. & Shaller, H. J. Virol. 64, 5324–5332 (1990).
Schlicht, H.-J., Radziwill, G. & Shaller, H. Cell 56, 85–92 (1989).
Coffin, J. M. in Fundamental Virology 2nd edn (eds Fields, B. N. et al.) 645–708 (Raven, New York, 1991).
Wickner, R. B. Cell 21, 217–226 (1980).
Fujimura, T. & Wickner, R. B. J. biol. Chem. 267, 2708–2713 (1992).
Valentine, R. C. & Green, N. M. J. molec. Biol. 27, 615–617 (1967).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fujimura, T., Ribas, J., Makhov, A. et al. Pol of gag–pol fusion protein required for encapsidation of viral RNA of yeast L-A virus. Nature 359, 746–749 (1992). https://doi.org/10.1038/359746a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/359746a0
This article is cited by
-
Expression of a synthetic rust fungal virus cDNA in yeast
Archives of Virology (2016)
-
Characterization of virus-like particles and identification of capsid proteins in Xanthophyllomyces dendrorhous
Virus Genes (2015)
-
Yeast viral killer toxins: lethality and self-protection
Nature Reviews Microbiology (2006)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.