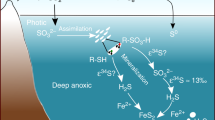
Abstract
THEORIES for the origin of life require the availability of reduced (or 'fixed') nitrogen-containing compounds, in particular ammonia. In reducing atmospheres, such compounds are readily formed by electrical discharges1,2, but geochemical evidence suggests that the early Earth had a non-reducing atmosphere1,3–6, in which discharges would have instead produced NO (refs 7–10). This would have been converted into nitric and nitrous acids and delivered to the early oceans as acid rain11. It is known12–15, however, that Fe(II) was present in the early oceans at much higher concentrations than are found today, and thus the oxidation of Fe(II) to Fe(III) provides a possible means for reducing nitrites and nitrates to ammonia. Here we explore this possibility in a series of experiments which mimic a broad range of prebiotic seawater conditions (the actual conditions on the early Earth remain poorly constrained). We find that the reduction by Fe(II) of nitrites and nitrates to ammonia could have been a significant source of reduced nitrogen on the early Earth, provided that the ocean pH exceeded 7.3 and is favoured for temperatures greater than about 25 °C.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Schopf, J. W. (ed.) Earth's Earliest Biosphere 53–92 (Princeton Univ. Press, 1983).
Stribling, R. & Miller, S. L. Orig. Life Evol. Biosph. 17, 261–273 (1978).
Walker, J. C. G. Orig. Life Evol. Biosph. 16, 117–127 (1985).
Mattioli, G. S. & Wood, B. J. Nature 322, 626–628 (1986).
Gregor, C. B., Garrels, R. M., Mackenzie, F. T. & Maynard, J. B. (eds) Chemical Cycles in the Evolution of the Earth 42–79 (Wiley, New York, 1988).
Kasting, J. F. Precambr. Res. 34, 205–229 (1987).
Chameides, W. L. & Walker, J. C. G. Orig. Life Evol. Biosph. 11, 291–302 (1981).
Yung, Y. L. & McElroy, M. B. Science 203, 1002–1004 (1979).
Kasting, J. F. Orig. Life Evol. Biosph. 20, 199–231 (1990).
Fegley, B. Jr, Prinn, R. G., Hartman, H. & Watkins, G. H. Nature 319, 305–308 (1986).
Mancinelli, R. L. & McKay, C. P. Orig. Life Evol. Biosph. 18, 311–325 (1988).
H. D. Holland Econ. Geol. 68, 1169–1172 (1973).
Walker, J. C. G. & Brimblecombe, P. Precambr. Res. 28, 205–222 (1985).
Derry, L. A. & Jacobsen, S. B. Geochim. Cosmochim. Acta 54, 2965–2975 (1990).
Holland, H. D. The Chemical Evolution of the Atmosphere and Oceans Ch. 4 (Princeton Univ. Press, 1989).
Buresh, R. J. & Moraghan, J. T. J. envir. Qual. 5, 320–324 (1976).
Walker, C. G. J. et al. in Earth's Earliest Biosphere (ed. Schopf, J. W.) 260–284 (Princeton Univ. Press, 1983).
Chyba, C. & Sagan, C. Orig. Life Evol. Biosph. 21, 3–17 (1991).
Grätzel, Von M., Taniguchi, S. & Henglein, A. Ber. Bunsenges. phys. Chem. 74, 1003–1010 (1970).
Doyle, M. P. & Mahapatro, S. N. J. Am. chem. Soc. 106, 3678–3679 (1984).
Butler, J. N. Carbon Dioxide Equilibria and Their Applications (Addison-Wesley, Reading, MA, 1982)
Freier, R. K. (ed.) Aqueous Solutions Vol 1 (de Gruyter, Berlin, 1976).
Holland, H. D. The Chemical Evolution of the Atmosphere and Oceans 205 (Princeton Univ. Press).
Windley, B. F. (ed.) The Early History of the Earth (Wiley, New York, 1976).
Turcotte, D. L. Earth planet. Sci. Lett. 48, 50–53 (1980).
Kasting, J. F., Zahnle, K. J., Pinto, J. P. & Young, A. T. Orig. Life Evol. Biosph. 19, 95–108 (1989).
Zafiriou, O. C. & True, M. B. Mar. Chem. 8, 9–32 (1979).
Broecker, W. S. & Peng, T.-H. Tracers in the Sea 243 (Lamont-Doherty geol. Obs. Palisades, 1982).
Kasting, J. F. J. geophys. Res. 87, 3091–3098 (1982).
Bada, J. L. & Miller, S. L. Science 159, 423–425 (1968).
Kuhn, W. E. & Kasting, J. F. Nature 301, 53–55 (1983).
Walker, C. G. J. Orig. Life Evol. Biosph. 16, 117–127 (1985).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Summers, D., Chang, S. Prebiotic ammonia from reduction of nitrite by iron (II) on the early Earth. Nature 365, 630–633 (1993). https://doi.org/10.1038/365630a0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/365630a0
This article is cited by
-
Isotopic constraints on lightning as a source of fixed nitrogen in Earth’s early biosphere
Nature Geoscience (2023)
-
Mineral-catalysed formation of marine NO and N2O on the anoxic early Earth
Nature Geoscience (2022)
-
Abiotic path of Archean nitrogen
Nature Geoscience (2022)
-
Plausible Emergence and Self Assembly of a Primitive Phospholipid from Reduced Phosphorus on the Primordial Earth
Origins of Life and Evolution of Biospheres (2021)
-
Impact-induced amino acid formation on Hadean Earth and Noachian Mars
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.