Abstract
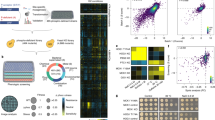
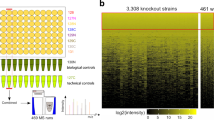
A large proportion of the 6,000 genes present in the genome of Saccharomyces cerevisiae, and of those sequenced in other organisms, encode proteins of unknown function. Many of these genes are “silent,” that is, they show no overt phenotype, in terms of growth rate or other fluxes, when they are deleted from the genome. We demonstrate how the intracellular concentrations of metabolites can reveal phenotypes for proteins active in metabolic regulation. Quantification of the change of several metabolite concentrations relative to the concentration change of one selected metabolite can reveal the site of action, in the metabolic network, of a silent gene. In the same way, comprehensive analyses of metabolite concentrations in mutants, providing “metabolic snapshots,” can reveal functions when snapshots from strains deleted for unstudied genes are compared to those deleted for known genes. This approach to functional analysis, using comparative metabolomics, we call FANCY—an abbreviation for functional analysis by co-responses in yeast.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Hieter, P. & Boguski, M. Functional genomics: It's all how you read it. Science 278, 601 –602 (1997).
Brent R. Genomic biology. Cell 10, 169–183 (2000).
Oliver, S.G. From DNA sequence to biological function. Nature 379 , 597–600 (1996).
Oliver, S. Guilt-by-association goes global. Nature 403, 601–603 (2000).
Oliver, S.G., Winson, M.K., Kell, D.B. & Baganz, F. Systematic functional analysis of the yeast genome. Trends Biotechnol. 16, 373–378 ( 1998).
Goffeau, A. et al. Life with 6000 genes. Science 274, 546, 563–7 (1996).
Teusink, B., Baganz, F., Westerhoff, H.V. & Oliver, S.G. Metabolic control analysis as a tool in the elucidation of the function of novel genes. Methods Microbiol. 26, 297– 336 (1998).
Smith, V., Chou, K.N., Lashkari, D., Botstein, D. & Brown, P.O. Functional analysis of the genes of yeast chromosome V by genetic footprinting. Science 274, 2069–2074 (1996).
Baganz F. et al. Quantitative analysis of yeast gene function using competition experiments in continuous culture. Yeast 14, 1417–1427 (1998).
Giaever, G. et al. Genomic profiling of drug sensitivities via induced haploinsufficiency . Nat. Genet. 21, 278–283 (1999).
Hofmeyr, J.H., Cornish-Bowden, A. & Rohwer, J.M. Taking enzyme kinetics out of control; putting control into regulation. Eur. J. Biochem. 212 , 833–837 (1993).
Hofmeyr, J.H. & Cornish-Bowden, A. Co-response analysis: a new experimental strategy for metabolic control analysis. J. Theoret. Biol. 182, 371–380 (1996).
Kholodenko, B.N., Schuster, S., Rohwer, J.M., Cascante, M. & Westerhoff, H.V. Composite control of cell function: metabolic pathways behaving as single control units. FEBS Lett. 368, 1–4 ( 1995).
Rohwer, J.M. Interaction of functional units in metabolism (Ph.D. Thesis). (University of Amsterdam, Amsterdam; 1997).
Rohwer, J.M., Schuster, S. & Westerhoff, H.V. How to recognize monofunctional units in a metabolic system. J. Theoret. Biol. 179, 213– 228 (1996).
Oliver, S.G. Yeast as a navigational aid in genome analysis. Microbiology 143, 1483–1487 (1997).
Kell, D.B. & Mendes, P. Snapshots of systems: metabolic control analysis and biotechnology in the post-genomic era. In Technological and medical implications of metabolic control analysis . (eds Cornish-Bowden, A. & Cárdenas, M.L.) 3– 25 (Kluwer Academic Publishers, Dordrecht; 2000).
Kretschmer, M. & Fraenkel, D.G. Yeast 6-phosphofructo-2-kinase: sequence and mutant. Biochemistry 30, 10663 –10672 (1991).
Kretschmer, M., Tempst, P. & Fraenkel, D.G. Identification and cloning of yeast phosphofructokinase 2. Eur. J. Biochem. 197, 367– 372 (1991).
Paravicini, G. & Kretschmer, M. The yeast FBP26 gene codes for a fructose-2,6-bisphosphatase. Biochemistry 31, 7126–7133 ( 1992).
Boles, E., Goehlmann, W.H. & Zimmermann, F.K. Cloning of a second gene encoding 6-phosphofructo-2-kinase in yeast, and its characterization of mutant strains without fructose-2,6-bisphosphate . Mol. Microbiol. 20, 65– 76 (1996).
Rousseau, G.G. & Hue, L. Mammalian 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase: a bifunctional enzyme that controls glycolysis. Prog. Nucleic Acids Res. Mol. Biol. 45, 99–127 (1993).
Van Schaftingen, E. Fructose 2,6-bisphosphate. Adv. Enzymol. Rel. Areas Mol. Biol. 59, 315–395 ( 1987).
Van Schaftingen, E., Lederer, B., Bartrons, R. & Hers, H.G. A kinetic study of pyrophosphate: fructose-6-phosphate phosphotransferase from potato tubers. Application to a microassay of fructose 2,6-bisphosphate . Eur. J. Biochem. 129, 191– 195 (1982).
Baganz, F., Hayes, A., Marren, D., Gardner, D.C.J. & Oliver, S.G. Suitability of replacement markers for functional analysis studies in Saccharomyces cerevisiae. Yeast 13, 1563–1573 ( 1997).
Wach, A., Brachat, A., Pöhlmann, R. & Philippsen, P. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast 10, 1793–1808 (1994).
Yocum, R. Genetic engineering of industrial yeasts. Proc. Bio Expo 86, 17 (1986).
Hutter, A. & Oliver, S.G. Ethanol production using nuclear petite yeast mutants. Appl. Microbiol. Biotechnol. 49, 511–516 ( 1998).
Griffiths, P.R. & de Haseth, J.A. Fourier transform infrared spectrometry. (Wiley, New York; 1986).
Winson, M.K. et al. Diffuse reflectance absorbance spectroscopy taking in chemometrics (DRASTIC). A hyperspectral FT-IR-based approach to rapid screening for metabolite overproduction. Analyt. Chim. Acta 348, 273–282 (1997).
Cole, R.B. Electrospray ionization mass spectrometry: fundamentals, instrumentation and applications. (Wiley, New York; 1997)
Gaskell, S.J. Electrospray: principles and practice. J. Mass Spec. 32, 677–688 (1997).
Lindon, J.C., Nicholson, J.K. & Wilson, I.D. Direct coupling of chromatographic separations to NMR spectroscopy. Prog. Nucl. Magn. Reson. Spectrosc. 29, 1–49 (1996).
Kacser, H. & Burns, J.A. The control of flux . Symp. Soc. Exp. Biol. 27, 65– 104 (1973).
Burns, J.A. et al. Control analysis of metabolic systems. Trends Biochem. Sci. 10, 16 (1985).
Kell, D.B. & Westerhoff, H.V. Metabolic control theory—its role in microbiology and biotechnology. FEMS Microbiol. Rev. 39, 305–320 ( 1986).
Fell, D.A. Understanding the control of metabolism. (Portland Press, London; 1996)
Cornish-Bowden, A. & Cardenas, M.L. From genome to cellular phenotype—a role for metabolic flux analysis? Nat. Biotechnol. 18, 267–268 (2000).
Heinrich, R. & Rapoport, T.A. Linear theory of enzymatic chains; its application for the analysis of the crossover theorem and of the glycolysis of human erythrocytes. Acta Biol. Med. Ger. 31, 479–494 ( 1973).
Oliver, S.G. Redundancy reveals drugs in action. Nat. Genet. 21, 245–246 (1999).
Arkin, A., Shen, P.D. & Ross, J. A test case of correlation metric construction of a reaction pathway from measurements. Science 277, 1275–1279 (1997).
Winzeler, E. et al. Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science 285 , 901–906 (1999).
Slonimski, P.P., Perrodin, G. & Croft, J.H. Ethidium bromide induced mutation of yeast mitochondria: complete transformation of cells into respiratory deficient non-chromosomal petites. Biochem. Biophys. Res. Commun. 30, 232–239 (1968).
Gonzalez, B., Francois, J. & Renaud, M. A rapid and reliable method for metabolite extraction in yeast using boiling buffered ethanol. Yeast 13, 1347–1355 (1997).
Bergmeyer, H.U. Methods of enzymatic analysis. (Verlag Chemiel, Basel; 1974)
Cornish-Bowden, A. & Hofmeyr, J.H. Determination of control coefficients in intact metabolic systems. Biochem. J. 298, 367–375 ( 1994).
Goodacre, R. et al. On mass spectrometer instrument standardization and interlaboratory calibration transfer using neural networks. Analyt. Chim. Acta 348, 511–532 ( 1997).
Goodacre, R. et al. Rapid identification of urinary tract infection bacteria using hyperspectral, whole organism fingerprinting and artificial neural networks . Microbiology 144, 1157– 1170 (1998).
Martens, H. & Næs, T. Multivariate calibration . (Wiley, Chichester; 1989).
Causton, D.R. A biologist's advanced mathematics. (London, Allen & Unwin; 1987).
Jolliffe, I.T. Principal components analysis. (Springer-Verlag, New York; 1986)
MacFie, H.J.H., Gutteridge, C.S. & Norris, J.R. Use of canonical variates in differentiation of bacteria by pyrolysis gas–liquid chromatography. J. Gen. Microbiol. 104, 67–74 ( 1978).
Windig,W., Haverkamp, J., & Kistemaker, P.G. Interpretation of sets of pyrolysis mass spectra by discriminant-analysis and graphical rotation. Analyt. Chem. 55, 81–88 (1983).
Winston, F., Dollard, C. & Ricupero-Hovasse, S.L. Construction of a set of convenient Saccharomyces cerevisiae strains that are isogenic to S288C. Yeast 11, 53–55 (1995).
Acknowledgements
This work was supported by EC contracts, within the frame of the EUROFAN program, to S.G.O., K.v.D., and H.V.W., and by a grant from the UK's Biotechnology and Biological Sciences Research Council to S.G.O. and D.B.K. We would like to thank Cathy Day for her superb technical assistance, and Barbara Bakker and Johann Rohwer for stimulating discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Raamsdonk, L., Teusink, B., Broadhurst, D. et al. A functional genomics strategy that uses metabolome data to reveal the phenotype of silent mutations. Nat Biotechnol 19, 45–50 (2001). https://doi.org/10.1038/83496
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/83496
This article is cited by
-
Quantitative LC–MS study of compounds found predictive of COVID-19 severity and outcome
Metabolomics (2023)
-
Saccharomyces cerevisiae does not undergo a quorum sensing-dependent switch of budding pattern
Scientific Reports (2022)
-
Untargeted metabolomics of COVID-19 patient serum reveals potential prognostic markers of both severity and outcome
Metabolomics (2022)
-
Empirical support for the biogeochemical niche hypothesis in forest trees
Nature Ecology & Evolution (2021)
-
Indication of high lipid content in epithelial-mesenchymal transitions of breast tissues
Scientific Reports (2021)