Abstract
Monocyte-derived dendritic cells (moDC) have been widely used in cancer immunotherapy but show significant donor-to-donor variability and low capacity for the cross-presentation of tumour-associated antigens (TAA) to CD8+ T cells, greatly limiting the success of this approach. Given recent developments in induced pluripotency and the relative ease with which induced pluripotent stem (iPS) cell lines may be generated from individuals, we have succeeded in differentiating dendritic cells (DC) from human leukocyte antigen (HLA)-A*0201+ iPS cells (iPS cell-derived DC (ipDC)), using protocols compliant with their subsequent clinical application. Unlike moDC, a subset of ipDC was found to coexpress CD141 and XCR1 that have been shown previously to define the human equivalent of mouse CD8α+ DC, in which the capacity for cross-presentation has been shown to reside. Accordingly, ipDC were able to cross-present the TAA, Melan A, to a CD8+ T-cell clone and stimulate primary Melan A-specific responses among naïve T cells from an HLA-A*0201+ donor. Given that CD141+XCR1+ DC are present in peripheral blood in trace numbers that preclude their clinical application, the ability to generate a potentially unlimited source from iPS cells offers the possibility of harnessing their capacity for cross-priming of cytotoxic T lymphocytes for the induction of tumour-specific immune responses.
Similar content being viewed by others
Introduction
The use of dendritic cells (DC) to prime responses to tumour-associated antigens (TAA) provides a promising approach to cancer immunotherapy,1 but clinically relevant responses have frequently been disappointing2, 3, 4 partly due to the properties of the DC commonly employed. Monocyte-derived DC (moDC) show significant donor-to-donor variation, frequently compounded by the side effects of ongoing chemotherapy. Furthermore, moDC display a limited capacity for cross-priming of antigen-specific CD8+ T cells, creating a dependence on exogenous peptides and further restricting the scope of such an approach to those human leukocyte antigen (HLA) haplotypes for which the immunodominant epitopes are known. As CD8α+ DC in mice are peculiarly capable of cross-presentation, the recent identification of CD141+XCR1+ DC as their functional equivalent in humans5, 6 has suggested that this subset may be better suited to the induction of antitumor responses.1, 7 Nevertheless, the trace number of such cells present in peripheral blood8 and the low yields obtained following the in vitro culture of cord blood progenitors,9 poses a substantial obstacle to their clinical application.10 Given the recent demonstration that human somatic cells may be reprogrammed to pluripotency,11, 12 we have developed protocols for the differentiation of CD141+XCR1+ DC from human-induced pluripotent stem (iPS) cells under conditions compliant with their subsequent clinical use. Our results demonstrate the feasibility of creating a potentially unlimited supply of autologous DC capable of the cross-presentation of TAA for cancer immunotherapy.
Results and discussion
Differentiation of clinical-grade DC from human iPS cells
The C15 and C19 iPS cell lines were derived from the dermal fibroblasts of an HLA-A*0201+ donor as described13 and were maintained long-term under feeder- and serum-free conditions. Undifferentiated cells displayed the morphology and high nucleus:cytoplasm ratio characteristic of human embryonic stem (ES) cells (Supplementary Figure 1a) and expressed the pluripotency-associated surface markers SSEA-4 and TRA1-60, together with the transcription factors Oct-4 and Nanog (Supplementary Figure 1b), known to be fundamental to the maintenance of pluripotency among ES cells. Human iPS cells formed conventional teratomas in immune-compromised mice13 and differentiated in vitro into embryoid bodies that, when plated onto tissue culture plastic, differentiated spontaneously into cell types derived from each of the three embryonic germ layers. Staining for the transcription factor SOX17 revealed endoderm commitment, while the presence of CD34+ hematopoietic cells provided evidence of mesoderm commitment and expression of βIII tubulin showed the presence of nascent neurons, consistent with ectoderm differentiation (Supplementary Figure 1c).
Although conventional DC have been differentiated previously from human iPS cells,14, 15 the reliance on animal products and the mouse OP-9 stromal cell line precludes their downstream clinical application. We therefore directed the differentiation of C19 towards the DC lineage by adapting protocols we had established previously for the differentiation of DC from human ES cells in the absence of animal products with the sole exception of matrigel, required for the routine passage of undifferentiated ES cells and iPS cells.16 After 19 days, cultures contained cells with distinctive dendritic morphology (Figure 1a), expressing CD11c and low levels of both MHC class II and CD40, suggestive of an immature phenotype (Figure 1b). iPS cell-derived DC (ipDC) could also be differentiated from C15, an independently derived iPS cell line, using the same protocol. Furthermore, although our original experiments were dependent on the use of matrigel for the routine passage of the iPS cell lines, C19 maintained on xeno-free Corning Synthemax culture plates (Corning, Lowell, MA, USA) were found to differentiate efficiently into ipDC, making our protocol fully compliant with clinical applications.
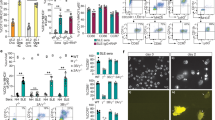
Characterisation of DC differentiated from human iPS cells. (a) Phase contrast micrograph showing a cluster of ipDC and the morphology of individual cells (inset). Scale bar: 40 μm. (b) Expression of DC-associated markers by ipDC (blue histograms) compared with isotype-matched controls (red histograms). (c) Comparison of the phenotype of ipDC and moDC following maturation with a cytokine cocktail. DC were gated as the CD11chi population and dead cells were excluded using 7-AAD. (d) ipDC stimulate proliferative responses among naïve allogeneic T cells. DC were mitomycin C (MMC) treated, washed and titrated into cultures of peripheral blood lymphocytes (PBL) from an allogeneic donor at a top stimulator:responder ratio of 1:10. Wells were pulsed with 3H-thymidine on day 4 and harvested 18 h later. Values of c.p.m. represent the average of triplicate cultures±s.d. Data are representative of three independent experiments.
To assess their capacity for maturation, ipDC were cultured with a maturation cocktail consisting of tumour necrosis factor-α, prostaglandin E2, interleukin (IL)-1β and interferon (IFN)-γ, shown to be effective for the maturation of DC differentiated from human ES cells.16 Following exposure to this maturation cocktail, ipDC secreted high concentrations of the pro-inflammatory cytokine IL-6 (Supplementary Figure 2a). Furthermore, upon maturation, ipDC lost expression of CD14 while upregulating MHC class II, CD83, conventional costimulatory molecules and the chemokine receptor, CCR7, yielding a phenotype similar to that of mature moDC (Figure 1c). Indeed, like their counterparts, ipDC stimulated potent proliferative responses among naïve allogeneic T cells in a dose-dependent manner (Figure 1d).
ipDC display the phenotype and function of cross-presenting DC
Despite these similarities, ipDC differed phenotypically from moDC: while they lacked significant expression of the plasmacytoid DC markers CD123 and BDCA-2, ipDC displayed a CD11bloCD141hi phenotype (Figure 1b), reminiscent of the recently described population of cross-presenting DC in human blood and secondary lymphoid organs.5, 6, 8, 9 Moreover, consistent with initial descriptions of this novel population, ipDC expressed intracellular Toll-like receptor (TLR) 3 (Figure 1b) and secreted high levels of IL-6 in response to poly(I:C), a specific agonist of TLR3, and the TLR7/8 agonist R848 (Supplementary Figures 2b and c). Interestingly, ipDC also expressed TLR9 (Figure 1b) and, unlike moDC, responded to its ligation by the CpG-containing oligodeoxynucleotide ODN2216 (Supplementary Figure 2d); although expression of TLR9 by CD141+ DC in human blood and lymphoid tissues was not detected, its expression by CD8α+ DC in the mouse has been described.10, 17 Furthermore, the ligation of TLR3 and TLR9 has been shown to specifically enhance cross-presentation of antigen by mouse DC.17, 18, 19
In the light of these findings, we investigated expression of the chemokine receptor XCR1. Although various DC populations are known to upregulate CD141 in culture,9 XCR1 expression has been shown to be highly specific for cross-presenting DC in both mouse and human, and to augment the antigen-driven expansion of CD8+ cytotoxic T lymphocytes.5, 6, 20 Although moDC failed to express this chemokine receptor, ipDC consistently contained a discrete population of cells coexpressing CD141 and XCR1 (Figure 2a). The CD141+XCR1− subset is highly reminiscent of DC differentiated from human ES cells,16 which, like moDC, upregulate CD141 in culture but consistently fail to express XCR1. Given that a subset of ipDC differs phenotypically from their human ES cell-derived counterparts, we investigated the capacity of ipDC to cross-present antigen using the melanoma-specific TAA, Melan A. When pulsed with the HLA-A*0201-restricted peptide from Melan A (Melan A26−35), both moDC and ipDC stimulated IFN-γ production by the CD8+ T-cell clone, 2D10, in a peptide concentration-dependent manner. However, when pulsed with recombinant Melan A protein as a source of unprocessed antigen, only ipDC were able to cross-present the Melan A26−35 epitope (Figure 2b) to the T-cell clone. Although the capacity for cross-presentation is most likely to reside within the XCR1+ subset of ipDC, our attempts to sort the population according to XCR1 expression consistently induced their apoptosis, making it impossible for us to formally exclude any contribution from the CD141+XCR1− subset. Nevertheless, although the exact identity of cross-presenting ipDC remains to be confirmed, our results strongly suggest the presence within our cultures of the human counterparts of murine CD8α+ DC.
Cross-presentation of TAA by ipDC. (a) Cultures of ipDC, but not moDC, contain a discrete population of cells coexpressing CD141 and XCR1. DC were gated on the CD11chi population. Data are representative of four independent experiments. (b) Whereas both HLA-A*0201+ ipDC and moDC pulsed with the Melan A26−35 peptide elicited IFN-γ production from the CD8+ T-cell clone 2D10 (top panel), when incubated with 1 μM Melan A protein only ipDC were able to cross-present the epitope to 2D10 (bottom panel). Bars represent the mean of replicate cultures±s.d. Statistical significance was calculated using an unpaired, two-tailed Student's t-test. *P<0.03; ns: not significant. Data are representative of three independent experiments.
Stimulation of primary TAA-specific responses by ipDC
To determine whether ipDC could prime TAA-specific responses among naïve T cells, we pulsed moDC and ipDC with Melan A26−35 peptide and cultured them with T cells depleted of CD45RO+ memory cells, purified from an HLA-A*0201+ donor. After 14 days, T cells were stained for CD8 and with HLA-A*0201-Melan A26−35 tetramers to detect antigen-specific T cells.21 Although both moDC and ipDC primed Melan A-specific CD8+ T cells when pulsed with the immunodominant epitope, only ipDC elicited a small but reproducible response when pulsed with whole Melan A protein, consistent with their capacity to cross-prime (Figure 3a). Importantly, T cells stimulated with antigen-pulsed moDC or ipDC upregulated IFN-γ, consistent with their activation (Figure 3b). Although we consistently showed the appearance of a modest population of antigen-specific T cells in co-cultures pulsed with whole Melan A, our data narrowly failed to reach statistical significance, most likely due to the expansion of residual alloreactive T cells and those specific for other Melan A-derived epitopes, thereby diluting the antigen-specific response.
Priming of Melan A-specific T-cell responses by ipDC. (a) ipDC cross-prime naïve Melan A-specific T cells from an HLA-A*0201+ donor. T cells were costained for CD8 and the relevant TCR using a HLA-A*0201-Melan A26−35 tetramer. (b) Priming of CD8+ T cells by ipDC elicits IFN-γ production. Following in vitro culture of naïve T cells with either unpulsed or Melan A26−35 pulsed DC, cells were restimulated with tetramer and peptide, and stained for surface CD8 and intracellular IFN-γ. Dead cells were excluded using 7-AAD staining. Data are representative of three independent experiments.
While identification of the human equivalent of murine CD8α+ DC offers promise for their use in cancer immunotherapy, their presence in peripheral blood at a frequency of 1:10 000 mononuclear cells9 has so far posed an insurmountable barrier to their clinical application.10 We have employed human iPS cells, capable of indefinite self-renewal, to generate potentially unlimited numbers of such cells in vitro, using protocols compatible with their future clinical use.16 Although significant progress has been made towards the establishment of a xeno-free culture system for the differentiation of conventional DC from human iPS cells, only certain iPS cell lines proved permissive, and viability was reported to be low.22 In contrast, our own protocols show no compromise of viability and are fully compatible with the generation of a subset of DC normally found in vivo in trace numbers. Although the preferred use of genetic modification for reprogramming of somatic cells to pluripotency currently remains an obstacle to the therapeutic use of ipDC, the advent of more efficient non-genetic approaches to reprogramming suggests that they may offer important clinical applications in the future.
The ongoing construction of extensive banks of clinically approved iPS cell lines, covering the common HLA haplotypes, may permit the matching of individual patients with lines expressing appropriate MHC restriction elements for the relevant TAA. Semi-allogeneic DC differentiated from mouse ES cells have been shown to stimulate potent antitumour responses in mice,23 indeed the stimulation of alloreactive T cells may provide a cytokine milieu conducive to the priming of naïve T cells specific for TAA.24 In this respect, the secretion of high levels of IL-6 by ipDC in response to the maturation cocktail, augurs well for breaking the regulatory T-cell barrier25, 26, 27 that constitutes one of the greatest obstacles to the induction of antitumour responses.1, 28 Although the selection of iPS cell lines from a pre-existing bank may prove to be the most pragmatic approach to their clinical application, where appropriate lines are not available, iPS cell technology offers the additional prospect of generating CD141+XCR1+ DC in a fully autologous manner.29
Materials and methods
Derivation and culture of human iPS cells
The C15 and C19 human iPS cell lines were derived and maintained, and their pluripotency assessed as described.13 Ethical approval was granted by the NHS National Research Ethics Service. Cells were cultured in six-well plates in a volume of 4 ml per well and the medium was replaced daily, except on the day following their passage. Before use, plates were coated with matrigel (BD Biosciences, Oxford, UK) diluted 1:30 and stored at 4 °C until use. Alternatively, Corning Synthemax Surface six-well plates were used for maintenance of the iPS cell lines, thereby avoiding the requirement for matrigel. Human iPS cells cultured in mTeSR1 (StemCell Technologies, Genoble, France) were routinely passaged every 6–7 days at a 1:12 dilution, while human iPS cells maintained in TeSR2 (StemCell Technologies) were passaged every 5–6 days at a dilution of 1:12–1:15.
Directed differentiation of DC
DC were differentiated from human iPS cells by adapting protocols described for human ES cells.16 Briefly, the differentiation medium consisted of XVIVO-15 (Lonza, Slough, UK) supplemented with non-essential amino acids, 2 mM L-glutamine, 1 mM sodium pyruvate (PAA Laboratories GmbH, Yeovil, UK), 5 × 10−5 M 2-mercaptoethanol (Sigma, Gillingham, UK), granulocyte-macrophage colony-stimulating factor (GM-CSF) (50 ng ml−1; Peprotech, London, UK), stem cell factor (20 ng ml−1; R&D Systems, Abingdon, UK), vascular endothelial growth factor (50 ng ml−1; Peprotech) and bone morphogenetic protein-4 (50 ng ml−1; R&D Systems) that were successively removed from cultures until only GM-CSF remained. Bone morphogenetic protein-4 was removed from day 5 onwards, followed by VEGF (day 14) and stem cell factor (day 19). On days 13–17 of culture, the medium was supplemented with 25 ng ml−1 of recombinant human IL-4 (Peprotech). The concentration of IL-4 in the medium was increased to 100 ng ml−1 as DC accumulated in the cultures (days 20–24). DC were harvested on days 24–28 using gentle pipetting. Each six-well plate yielded, on average, 7.2±0.3 × 105 (s.e.m.) ipDC that were passed through a 70-μm cell strainer (BD Falcon, Oxford, UK) and plated at 5 × 105–1 × 106 per well of a six-well CellBind plate (Corning) in complete XVIVO-15 medium containing 50 ng ml−1 GM-CSF and 100 ng ml−1 IL-4. After 2–4 days, DC were matured for the final 48 h of culture as described,16 with a cocktail of cytokines consisting of tumour necrosis factor-α (50 ng ml−1; R&D Systems), prostaglandin E2 (1 μg ml−1; Sigma), IL-1β (10 ng ml−1; R&D Systems) and IFN-γ (20 ng ml−1; R&D Systems).
Isolation and culture of primary cells
Monocytes and naïve T cells were isolated from the peripheral blood mononuclear cells of buffy coats (NHS blood transfusion service) or the fresh blood of volunteers, under informed consent, using CD14-coated beads or a pan T-cell selection kit followed by depletion of CD45RO+ memory cells using the relevant selection kits/beads (Miltenyi Biotech, Woking, UK). CD4+ T cells were not excluded from the responder population in order to provide a potential source of T-cell help. AutoMACs separation was used to either positively select or deplete labelled populations of peripheral blood mononuclear cells, according to the manufacturer's instructions. Following removal of CD14+ monocytes, peripheral blood lymphocytes were cultured in Roswell Park Memorial Institute medium (RPMI) supplemented with 10% fetal calf serum, 50 U ml−1 penicillin, 50 μg ml−1 streptomycin and 2 mM L-glutamine (PAA Laboratories GmbH) (R10). MoDC were differentiated by culturing CD14+ monocytes in R10 containing 50 ng ml−1 GM-CSF and 100 ng ml−1 IL-4 for 6–8 days. Although the resulting moDC upregulated CD141 in culture, we were consistently unable to detect XCR1 expression at either the mRNA or protein level, even after prolonged culture for 14 days. This phenotype is consistent with the inability of moDC to cross-present antigen and endorses their use as a control population.
In vitro priming of naive antigen-specific T cells
Harvested HLA-A*0201+ moDC and ipDC were either untreated or pulsed with 1 μM Melan A26−35 peptide for 2½–3 h and washed. DC were plated at 4 × 104 per well of a 48-well plate or 1 × 105 per well of a 24-well plate, and unpulsed cells were either treated with 1 μM whole Melan A protein or left untreated. DC were cultured with HLA-A*0201-restricted naïve T cells from a different donor to the moDC to yield a ratio of 1:10 DC:T cells. Cells were cultured at 37 °C, 5% CO2 for 13–14 days in RPMI containing 5% human male AB serum (Sigma), 50 U ml−1 penicillin, 50 μg ml−1 streptomycin and 2 mM L-glutamine. Recombinant human IL-2 was added at 10 U ml−1 from days 4–7, and T cells were expanded using 500 U ml−1 recombinant human IL-2 for the remainder of the culture period.
Flow cytometry
DC or human iPS cells were incubated for 15 min on ice in blocking buffer (phosphate-buffered saline, 5% normal rabbit serum 0.5% bovine serum albumin, 0.1% NaN3) and washed twice. Cells were stained on ice in phosphate-buffered saline/2% fetal calf serum for 30 min with one of the following antibodies: TRA1-60 (Millipore, Watford, UK), SSEA-4 (clone: MC-813-70, R&D Systems), HLA-A2 (BB7.2), CD11c (BU15), CD11b (ICRF44), HLA-DR/DQ/DP (WR18), CD40 (LOB7/6) (AbD Serotec, Kidlington, UK), CD123 (9F5, BD Pharmingen, Oxford, UK), BDCA-4 (446921), BDCA-2 (polyclonal goat IgG), BDCA-3 (501733) (R&D Systems), CD83 (HB15e), CD80 (MEM-233), CD86 (BU63), CD14 (MEM18), CD54 (15.2, AbD Serotec), CCR7 (3D12, eBioscience, Hatfield, UK), XCR1 (polyclonal goat IgG; R&D Systems). For the final 10 min, 7-AAD was added at a concentration of 250 ng ml−1. Cells were washed twice and fixed in 2% formaldehyde.
Intranuclear staining was performed using commercial permeabilisation and fixation buffers (eBioscience), according to the manufacturer's instructions together with antibodies specific for the transcription factors Oct-4 (240408) or Nanog (polyclonal goat IgG; R&D Systems). T cells were labelled with tetramer as described21 and stained for intracellular cytokines. Briefly, T cells were first labelled with HLA-A*0201-MelanA26−35 tetramer and were untreated or stimulated with either 20 μM Melan A26−35 peptide or 50 ng ml−1 PMA and 500 μg ml−1 ionomycin (Sigma) for 6 h, as a positive control. For the final 4 h of culture, 10 μg ml−1 Brefeldin A (Sigma) was added. Cells were washed, surface stained for CD8 and fixed in 2% paraformaldehyde. Buffer containing saponin (phosphate-buffered saline, 0.5% saponin, 0.5% bovine serum albumin, NaN3; Sigma) was used to wash cells twice before staining for IFN-γ (25723, R&D Systems). Cells were analysed on a Beckton Dickinson FACSCalibur (BD Biosciences).
Antigen processing and presentation
DC were harvested and either untreated or fixed with 0.5% paraformaldehyde for 10 min at room temperature. DC were plated at 1–1.5 × 104 cells per well in a 96-well flat-bottomed plate. Fixed or unfixed cells were left untreated or incubated with either 1 μM Melan A (AMS Biotechnology, Abingdon, UK) or, as a positive control for antigen presentation, pulsed with either 10 nM or 1 μM Melan A26−35 peptide (ELAGIGILTV).21 The CD8+ T-cell clone, 2D10, which is specific for the HLA-A*0201-Melan A26−35 complex was plated at a 1:5 ratio of DC:T cells. Co-cultures were incubated at 37 °C, 5% CO2 for 40 h, before supernatants were harvested.
Enzyme-linked immunosorbent assays
IFN-γ and IL-6 ready-SET-go enzyme-linked immunosorbent assay (ELISA) kits and immunosorb plates were purchased from eBioscience and ELISAs were performed according to the manufacturer's instructions. ELISAs were read at 450 nm using a BioTek ELX808 plate reader (BioTeK, Potton, UK).
References
Palucka K, Banchereau J, Mellman I . Designing vaccines based on biology of human dendritic cell subsets. Immunity 2010; 33: 464–478.
Engell-Noerregaard L, Hansen TH, Andersen MH, Straten P, Svane IM . Review of clinical studies on dendritic cell-based vaccination of patients with malignant melanoma: assessment of correlation between clinical response and vaccine parameters. Cancer Immunol Immunother 2009; 58: 1–14.
Rosenberg SA, Sherry RM, Morton KE, Scharfman WJ, Yang JC, Topalian SL et al. Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J Immunol 2005; 175: 6169–6176.
Robson NC, Hoves S, Maraskovsky E, Schnurr M . Presentation of tumour antigens by dendritic cells and challenges faced. Curr Opin Immunol 2010; 22: 137–144.
Bachem A, Güttler S, Hartung E, Ebstein F, Schaefer M, Tannert A et al. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J Exp Med 2010; 207: 1273–1281.
Crozat K, Guiton R, Contreras V, Feuillet V, Dutertre C-A, Ventre E et al. The XC chemokine receptor 1 is a conserved selective marker of mammalian cells homologous to mouse CD8α+ dendritic cells. J Exp Med 2010; 207: 1283–1292.
Gallois A, Bhardwaj N . A needle in the ‘cancer vaccine’ haystack. Nat Med 2010; 16: 854–856.
Jongbloed SL, Kassianos AJ, McDonald KJ, Clark GJ, Ju X, Angel CE et al. Human CD141+(BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J Exp Med 2010; 207: 1247–1260.
Poulin LF, Salio M, Griessinger E, Anjos-Afonso F, Cracium L, Chen J-L et al. Characterization of human DNGR-1+ BDCA3+ leukocytes as putative equivalents of mouse CD8α+ dendritic cells. J Exp Med 2010; 207: 1261–1271.
Villadangos JA, Shortman K . Found in translation: the human equivalent of mouse CD8+ dendritic cells. J Exp Med 2010; 207: 1131–1134.
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007; 131: 861–872.
Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature 2007; 451: 141–146.
Carpenter L, Malladi R, Yang C-T, French A, Pilkington KJ, Forsey RW et al. Human induced pluripotent stem cells are capable of B-cell lymphopoiesis. Blood 2011; 117: 4008–4011.
Choi K-D, Vodyanik MA, Slukvin II . Generation of mature human myelomonocytic cells through expansion and differentiation of pluripotent stem cell-derived lin−CD34+CD43+CD45+ progenitors. J Clin Invest 2009; 119: 2818–2829.
Choi K-D, Vodyanik M, Slukvin II . Hematopoietic differentiation and production of mature myeloid cells from human pluripotent stem cells. Nat Protoc 2011; 6: 296–313.
Tseng S-Y, Nishimoto KP, Silk KM, Majumdar AS, Dawes GN, Waldmann H et al. Generation of immunogenic dendritic cells from human embryonic stem cells without serum and feeder cells. Regen Med 2009; 4: 513–526.
de Brito C, Tomkowiak M, Ghittoni R, Caux C, Leverrier Y, Marvel J . CpG promotes cross-presentation of dead cell-associated antigens by pre-CD8α+ dendritic cells. J Immunol 2011; 186: 1503–1511.
Maurer T, Heit A, Hochrein H, Ampenberger F, O’Keeffe M, Bauer S et al. CpG-DNA aided cross-presentation of soluble antigens by dendritic cells. Eur J Immunol 2002; 32: 2356–2364.
Schulz O, Diebold SS, Chen M, Naslund TI, Nolte MA, Alexopoulou L et al. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature 2005; 433: 887–892.
Dorner BG, Dorner MB, Zhou X, Opitz C, Mora A, Güttler S et al. Selective expression of the chemokine receptor XCR1 on cross-presenting dendritic cells determines cooperation with CD8+ T cells. Immunity 2009; 31: 823–833.
Romero P, Dunbar PR, Valmori D, Pittet M, Ogg GS, Rimoldi D et al. Ex vivo staining of metastatic lymph nodes by class I major histocompatibility complex tetramers reveals high numbers of antigen-experienced tumor-specific cytolytic T lymphocytes. J Exp Med 1998; 188: 1641–1650.
Senju S, Haruta M, Matsumura K, Matsunaga S, Fukushima S, Ikeda T et al. Generation of dendritic cells and macrophages from human induced pluripotent stem cells aiming at cell therapy. Gene Ther 2011; 18: 874–883.
Fukuma D, Matsuyoshi H, Hirata S, Kurisaki A, Motomura Y, Yoshitake Y et al. Cancer prevention with semi-allogeneic ES cell-derived dendritic cells. Biochem Biophys Res Commun 2005; 335: 5–13.
Fabre JW . The allogeneic response and anti-tumor immunity. Nat Med 2001; 7: 649–652.
Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity 2008; 29: 44–56.
Sharma MD, Hou D-Y, Liu Y, Koni PA, Metz R, Chandler P et al. Indoleamine 2,3-dioxygenase controls conversion of Foxp3+ Tregs to TH17-like cells in tumor-draining lymph nodes. Blood 2009; 113: 6102–6111.
Kimura A, Kishimoto T . IL-6: regulator of Treg/Th17 balance. Eur J Immunol 2010; 40: 1830–1835.
Zhou G, Drake CG, Levitsky HI . Amplification of tumor-specific regulatory T cells following therapeutic cancer vaccines. Blood 2006; 107: 628–636.
Nishikawa S, Goldstein RA, Nierras CR . The promise of human induced pluripotent stem cells for research and therapy. Nature Rev Mol Cell Biol 2008; 9: 725–729.
Acknowledgements
This work was supported by Grant G0802538 from the Medical Research Council (UK) and seed funding from the Oxford Stem Cell Institute (PJF), the Cancer Research UK programme Grant C399/A2291 (VC) and the NHS Blood and Transplant (LC and SMW), and presents independent research commissioned by the National Institute for Health Research (NIHR) under its programme grant scheme. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Gene Therapy website
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Silk, K., Silk, J., Ichiryu, N. et al. Cross-presentation of tumour antigens by human induced pluripotent stem cell-derived CD141+XCR1+ dendritic cells. Gene Ther 19, 1035–1040 (2012). https://doi.org/10.1038/gt.2011.177
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2011.177
Keywords
This article is cited by
-
CD8α+ dendritic cells potentiate antitumor and immune activities against murine ovarian cancers
Scientific Reports (2023)
-
Induced pluripotent stem cell-derived dendritic cell vaccine therapy genetically modified on the ubiquitin-proteasome system
Gene Therapy (2023)
-
Tumor RNA transfected DCs derived from iPS cells elicit cytotoxicity against cancer cells induced from colorectal cancer patients in vitro
Scientific Reports (2022)
-
Single-cell map of diverse immune phenotypes in the acute myeloid leukemia microenvironment
Biomarker Research (2021)
-
Cancer Vaccine Therapy Using Carcinoembryonic Antigen - expressing Dendritic Cells generated from Induced Pluripotent Stem Cells
Scientific Reports (2018)