Abstract
Background:
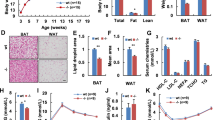
In the central nervous system (CNS), thyrotropin-releasing hormone (TRH) has an important role in regulating energy balance. We previously showed that dietary deprivation of leucine in mice increases energy expenditure through CNS-dependent regulation. However, the involvement of central TRH in this regulation has not been reported.
Methods:
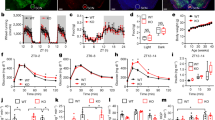
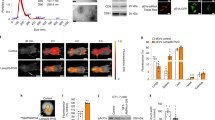
Male C57J/B6 mice were maintained on a control or leucine-deficient diet for 7 days. Leucine-deprived mice were either third intracerebroventricular (i.c.v.) injected with a TRH antibody followed by intraperitoneal (i.p.) injection of triiodothyronine (T3) or i.c.v. administrated with an adenovirus of shCREB (cAMP-response element binding protein) followed by i.c.v. injection of TRH. Food intake and body weight were monitored daily. Oxygen consumption, physical activity and rectal temperature were assessed after the treatment. After being killed, the hypothalamus and the brown adipose tissue were collected and the expression of related genes and proteins related was analyzed. In other experiments, control or leucine-deficient medium incubated primary cultured neurons were either infected with adenovirus-mediated short hairpin RNA targeting extracellular signal-regulated kinases 1 and 2 (Ad-shERK1/2) or transfected with plasmid-overexpressing protein phosphatase 1 regulatory subunit 3C (PPP1R3C).
Results:
I.c.v. administration of anti-TRH antibodies significantly reduced leucine deprivation-stimulated energy expenditure. Furthermore, the effects of i.c.v. TRH antibodies were reversed by i.p. injection of T3 during leucine deprivation. Moreover, i.c.v. injection of Ad-shCREB (adenovirus-mediated short hairpin RNA targeting CREB) significantly suppressed leucine deprivation-stimulated energy expenditure via modulation of TRH expression. Lastly, TRH expression was regulated by CREB, which was phosphorylated by ERK1/2 and dephosphorylated by PPP1R3C-containing protein Ser/Thr phosphatase type 1 (PP1) under leucine deprivation in vitro.
Conclusions:
Our data indicate a novel role for TRH in regulating energy expenditure via T3 during leucine deprivation. Furthermore, our findings reveal that TRH expression is activated by CREB, which is phosphorylated by ERK1/2 and dephosphorylated by PPP1R3C-containing PP1. Collectively, our studies provide novel insights into the regulation of energy homeostasis by the CNS in response to an essential amino-acid deprivation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 2009; 9: 311–326.
Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E et al. Metabolite profiles and the risk of developing diabetes. Nat Med 2011; 17: 448–453.
Cheng Y, Meng Q, Wang C, Li H, Huang Z, Chen S et al. Leucine deprivation decreases fat mass by stimulation of lipolysis in white adipose tissue and upregulation of uncoupling protein 1 (UCP1) in brown adipose tissue. Diabetes 2010; 59: 17–25.
Cheng Y, Zhang Q, Meng Q, Xia T, Huang Z, Wang C et al. Leucine deprivation stimulates fat loss via increasing CRH expression in the hypothalamus and activating the sympathetic nervous system. Mol Endocrinol (Baltimore, MD) 2012; 25: 1624–1635.
Guo F, Cavener DR . The GCN2 eIF2alpha kinase regulates fatty-acid homeostasis in the liver during deprivation of an essential amino acid. Cell Metab 2007; 5: 103–114.
Xia T, Cheng Y, Zhang Q, Xiao F, Liu B, Chen S et al. S6K1 in the central nervous system regulates energy expenditure via MC4R/CRH pathways in response to deprivation of an essential amino acid. Diabetes 2012; 61: 2461–2471.
Kilberg MS, Balasubramanian M, Fu L, Shan J . The transcription factor network associated with the amino acid response in mammalian cells. Adv Nutr (Bethesda, MD) 2012; 3: 295–306.
Hinnebusch AG . The eIF-2 alpha kinases: regulators of protein synthesis in starvation and stress. Sem Cell Biol 1994; 5: 417–426.
Kilberg MS, Pan YX, Chen H, Leung-Pineda V . Nutritional control of gene expression: how mammalian cells respond to amino acid limitation. Annu Rev Nutr 2005; 25: 59–85.
Wek RC . eIF-2 kinases: regulators of general and gene-specific translation initiation. Trends Biochem Sci 1994; 19: 491–496.
White BD, He B, Dean RG, Martin RJ . Low protein diets increase neuropeptide Y gene expression in the basomedial hypothalamus of rats. J Nutr 1994; 124: 1152–1160.
Du F, Higginbotham DA, White BD . Food intake, energy balance and serum leptin concentrations in rats fed low-protein diets. J Nutr 2000; 130: 514–521.
Porrini M, Santangelo A, Crovetti R, Riso P, Testolin G, Blundell JE . Weight, protein, fat, and timing of preloads affect food intake. Physiol Behav 1997; 62: 563–570.
Castaneda C, Charnley JM, Evans WJ, Crim MC . Elderly women accommodate to a low-protein diet with losses of body cell mass, muscle function, and immune response. Am J Clin Nutr 1995; 62: 30–39.
Hill JO, Melanson EL, Wyatt HT . Dietary fat intake and regulation of energy balance: implications for obesity. J Nutr 2000; 130: 284S–288S.
Blouet C, Schwartz GJ . Hypothalamic nutrient sensing in the control of energy homeostasis. Behav Brain Res 2010; 209: 1–12.
Lechan RM, Wu P, Jackson IM . Immunolocalization of the thyrotropin-releasing hormone prohormone in the rat central nervous system. Endocrinology 1986; 119: 1210–1216.
Hall R, Amos J, Garry R, Buxton RL . Thyroid-stimulating hormone response to synthetic thyrotrophin releasing hormone in man. BMJ 1970; 2: 274–277.
Harris AR, Christianson D, Smith MS, Fang SL, Braverman LE, Vagenakis AG . The physiological role of thyrotropin-releasing hormone in the regulation of thyroid-stimulating hormone and prolactin secretion in the rat. J Clin Invest 1978; 61: 441–448.
Nillni EA . Regulation of the hypothalamic thyrotropin releasing hormone (TRH) neuron by neuronal and peripheral inputs. Front Neuroendocrinol 2010; 31: 134–156.
Hollenberg AN . The role of the thyrotropin-releasing hormone (TRH) neuron as a metabolic sensor. Thyroid 2008; 18: 131–139.
Lechan RM, Fekete C . The TRH neuron: a hypothalamic integrator of energy metabolism. Progr Brain Res 2006; 153: 209–235.
Cote-Velez A, Perez-Martinez L, Charli JL, Joseph-Bravo P . The PKC and ERK/MAPK pathways regulate glucocorticoid action on TRH transcription. Neurochem Res 2008; 33: 1582–1591.
Harris M, Aschkenasi C, Elias CF, Chandrankunnel A, Nillni EA, Bjoorbaek C et al. Transcriptional regulation of the thyrotropin-releasing hormone gene by leptin and melanocortin signaling. J Clin Invest 2001; 107: 111–120.
Bianco AC, Kim BW . Deiodinases: implications of the local control of thyroid hormone action. J Clin Invest 2006; 116: 2571–2579.
Sarkar S, Legradi G, Lechan RM . Intracerebroventricular administration of alpha-melanocyte stimulating hormone increases phosphorylation of CREB in TRH- and CRH-producing neurons of the hypothalamic paraventricular nucleus. Brain Res 2002; 945: 50–59.
Cote-Vélez A, Pérez-Maldonado A, Osunab J, Barreraa B, Charlia J-L, Creb Joseph-Bravo P . and Sp/Krüppel response elements cooperate to control rat TRH gene transcription in response to cAMP. Biochim Biophys Acta (BBA) 2011; 1809: 191–199.
Xing J, Ginty DD, Greenberg ME . Coupling of the RAS-MAPK pathway to gene activation by RSK2, a growth factor-regulated CREB kinase. Science (New York, NY) 1996; 273: 959–963.
Shima H, Tohda H, Aonuma S, Nakayasu M, DePaoli-Roach AA, Sugimura T et al. Characterization of the PP2A alpha gene mutation in okadaic acid-resistant variants of CHO-K1 cells. Proc Natl Acad Sci USA 1994; 91: 9267–9271.
Wadzinski BE, Wheat WH, Jaspers S, Peruski LF Jr, Lickteig RL, Johnson GL et al. Nuclear protein phosphatase 2A dephosphorylates protein kinase A-phosphorylated CREB and regulates CREB transcriptional stimulation. Mol Cell Biol 1993; 13: 2822–2834.
Luo X, Zhang Y, Ruan X, Jiang X, Zhu L, Wang X et al. Fasting-induced protein phosphatase 1 regulatory subunit contributes to postprandial blood glucose homeostasis via regulation of hepatic glycogenesis. Diabetes 2011; 60: 1435–1445.
Tamura Y, Shintani M, Nakamura A, Monden M, Shiomi H . Phase-specific central regulatory systems of hibernation in Syrian hamsters. Brain Res 2005; 1045: 88–96.
Diop L, Pascaud X, Junien JL, Bueno L . CRF triggers the CNS release of TRH in stress-induced changes in gastric emptying. Am J Physiol 1991; 260: G39–G44.
Crupi R, Paterniti I, Campolo M, Di Paola R, Cuzzocrea S, Esposito E . Exogenous T3 administration provides neuroprotection in a murine model of traumatic brain injury. Pharmacol Res 2013; 70: 80–89.
Sanchez VC, Goldstein J, Stuart RC, Hovanesian V, Huo L, Munzberg H et al. Regulation of hypothalamic prohormone convertases 1 and 2 and effects on processing of prothyrotropin-releasing hormone. J Clin Invest 2004; 114: 357–369.
Harper ME, Seifert EL . Thyroid hormone effects on mitochondrial energetics. Thyroid 2008; 18: 145–156.
Hernandez DE, Arredondo ME, Xue BG, Jennes L . Evidence for a role of brain thyrotropin-releasing hormone (TRH) on stress gastric lesion formation in rats. Brain Res Bull 1990; 24: 693–695.
Garcia SI, Dabsys SM, Martinez VN, Delorenzi A, Santajuliana D, Nahmod VE et al. Thyrotropin-releasing hormone hyperactivity in the preoptic area of spontaneously hypertensive rats. Hypertension 1995; 26: 1105–1110.
Coll AP, Farooqi IS, O'Rahilly S . The hormonal control of food intake. Cell 2007; 129: 251–262.
Shaywitz AJ, Greenberg ME . CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem 1999; 68: 821–861.
Doherty MJ, Young PR, Cohen PT . Amino acid sequence of a novel protein phosphatase 1 binding protein (R5) which is related to the liver- and muscle-specific glycogen binding subunits of protein phosphatase 1. FEBS Lett 1996; 399: 339–343.
Alkemade A . Central and peripheral effects of thyroid hormone signalling in the control of energy metabolism. J Neuroendocrinol 2009; 22: 56–63.
Bachman ES, Dhillon H, Zhang CY, Cinti S, Bianco AC, Kobilka BK et al. betaAR signaling required for diet-induced thermogenesis and obesity resistance. Science (New York, NY) 2002; 297: 843–845.
Ohki-Hamazaki H, Watase K, Yamamoto K, Ogura H, Yamano M, Yamada K et al. Mice lacking bombesin receptor subtype-3 develop metabolic defects and obesity. Nature 1997; 390: 165–169.
Guo F, Bakal K, Minokoshi Y, Hollenberg AN . Leptin signaling targets the thyrotropin-releasing hormone gene promoter in vivo. Endocrinology 2004; 145: 2221–2227.
Lee SL, Stewart K, Goodman RH . Structure of the gene encoding rat thyrotropin releasing hormone. J Biol Chem 1988; 263: 16604–16609.
Valverde O, Mantamadiotis T, Torrecilla M, Ugedo L, Pineda J, Bleckmann S et al. Modulation of anxiety-like behavior and morphine dependence in CREB-deficient mice. Neuropsychopharmacology 2004; 29: 1122–1133.
Silva AJ, Kogan JH, Frankland PW, Kida S . CREB and memory. Annu Rev Neurosci 1998; 21: 127–148.
Mantamadiotis T, Lemberger T, Bleckmann SC, Kern H, Kretz O, Martin Villalba A et al. Disruption of CREB function in brain leads to neurodegeneration. Nat Genet 2002; 31: 47–54.
Chiappini F, Cunha LL, Harris JC, Hollenberg AN . Lack of cAMP-response element-binding protein 1 in the hypothalamus causes obesity. J Biol Chem 2011; 286: 8094–8105.
Chiappini F, Ramadoss P, Vella KR, Cunha LL, Ye FD, Stuart RC et al. Family members CREB and CREM control thyrotropin-releasing hormone (TRH) expression in the hypothalamus. Mol Cell Endocrinol 2013; 365: 84–94.
Barford D, Das AK, Egloff MP . The structure and mechanism of protein phosphatases: insights into catalysis and regulation. Annu Rev Biophys Biomol Struct 1998; 27: 133–164.
Goldberg J, Huang HB, Kwon YG, Greengard P, Nairn AC, Kuriyan J . Three-dimensional structure of the catalytic subunit of protein serine/threonine phosphatase-1. Nature 1995; 376: 745–753.
Price NE, Mumby MC . Effects of regulatory subunits on the kinetics of protein phosphatase 2A. Biochemistry 2000; 39: 11312–11318.
Rahmouni K, Sigmund CD, Haynes WG, Mark AL . Hypothalamic ERK mediates the anorectic and thermogenic sympathetic effects of leptin. Diabetes 2009; 58: 536–542.
Schrauwen P, Westerterp KR . The role of high-fat diets and physical activity in the regulation of body weight. Br J Nutr 2000; 84: 417–427.
Hao S, Sharp JW, Ross-Inta CM, McDaniel BJ, Anthony TG, Wek RC et al. Uncharged tRNA and sensing of amino acid deficiency in mammalian piriform cortex. Science (New York, NY) 2005; 307: 1776–1778.
Acknowledgements
This work was supported by grants from the Ministry of Science and Technology of China (973 Program 2010CB912502); National Natural Science Foundation (81130076, 31271269, 81100615, 81300659, 81390350 and 81325005); the Key Program of Shanghai Scientific and Technological Innovation Action Plan (13JC1409000); International S&T Cooperation Program of China (Singapore 2014DFG32470). FG was also supported by the One Hundred Talents Program of the Chinese Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Supplementary information
Rights and permissions
About this article
Cite this article
Xia, T., Zhang, Q., Xiao, Y. et al. CREB/TRH pathway in the central nervous system regulates energy expenditure in response to deprivation of an essential amino acid. Int J Obes 39, 105–113 (2015). https://doi.org/10.1038/ijo.2014.65
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2014.65
Keywords
This article is cited by
-
Effects of postnatal overfeeding and fish oil diet on energy expenditure in rats
Pediatric Research (2018)
-
Caffeine inhibits hypothalamic A1R to excite oxytocin neuron and ameliorate dietary obesity in mice
Nature Communications (2017)