Abstract
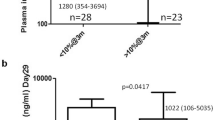
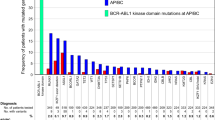
Tyrosine kinase inhibitor (TKI) therapy results in excellent responses in the majority of patients with chronic myeloid leukaemia. First-line imatinib treatment, with selective switching to nilotinib when patients fail to meet specific molecular targets or for imatinib intolerance, results in excellent overall molecular responses and survival. However, this strategy is less effective in cases of primary imatinib resistance; moreover, 25% of patients develop secondary resistance such that 20–35% of patients initially treated with imatinib will eventually experience treatment failure. Early identification of these patients is of high clinical relevance. Since the drug efflux transporter ABCB1 has previously been implicated in TKI resistance, we determined if early increases in ABCB1 mRNA expression (fold change from diagnosis to day 22 of imatinib therapy) predict for patient response. Indeed, patients exhibiting a high fold rise (⩾2.2, n=79) were significantly less likely to achieve early molecular response (BCR-ABL1IS ⩽10% at 3 months; P=0.001), major molecular response (P<0.0001) and MR4.5 (P<0.0001). Additionally, patients demonstrated increased levels of ABCB1 mRNA before the development of mutations and/or progression to blast crisis. Patients with high fold rise in ABCB1 mRNA were also less likely to achieve major molecular response when switched to nilotinib therapy (49% vs 82% of patients with low fold rise). We conclude that early evaluation of the fold change in ABCB1 mRNA expression may identify patients likely to be resistant to first- and second-generation TKIs and who may be candidates for alternative therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Nowell PC, Hungerford DA . A minute chromosome in human chronic granulocytic leukemia. Science 1960; 132: 1497.
Rowley JD . Letter: a new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature 1973; 243: 290–293.
Druker BJ, Tamura S, Buchdunger E, Ohno S, Segal GM, Fanning S et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med 1996; 2: 561–566.
Giles FJ, le Coutre PD, Pinilla-Ibarz J, Larson RA, Gattermann N, Ottmann OG et al. Nilotinib in imatinib-resistant or imatinib-intolerant patients with chronic myeloid leukemia in chronic phase: 48-month follow-up results of a phase II study. Leukemia 2013; 27: 107–112.
Khorashad JS, Kelley TW, Szankasi P, Mason CC, Soverini S, Adrian LT et al. BCR-ABL1 compound mutations in tyrosine kinase inhibitor-resistant CML: frequency and clonal relationships. Blood 2013; 121: 489–498.
Deininger M, O'Brien SG, Guilhot F, Goldman JM, Hochhaus A, Hughes TP et al. International Randomized Study of Interferon vs STI571 (IRIS) 8-Year Follow Up: sustained survival and low risk for progression or events in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib. ASH Annu Meet Abstr 2009; 114: 1126.
Hochhaus A, Saglio G, Larson RA, Kim DW, Etienne G, Rosti G et al. Nilotinib is associated with a reduced incidence of BCR-ABL mutations vs imatinib in patients with newly diagnosed chronic myeloid leukemia in chronic phase. Blood 2013; 121: 3703–3708.
Kantarjian H, Shah NP, Hochhaus A, Cortes J, Shah S, Ayala M et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 2010; 362: 2260–2270.
Saglio G, Kim DW, Issaragrisil S, le Coutre P, Etienne G, Lobo C et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med 2010; 362: 2251–2259.
Branford S, Rudzki Z, Walsh S, Parkinson I, Grigg A, Szer J et al. Detection of BCR-ABL mutations in patients with CML treated with imatinib is virtually always accompanied by clinical resistance, and mutations in the ATP phosphate-binding loop (P-loop) are associated with a poor prognosis. Blood 2003; 102: 276–283.
Hughes T, Saglio G, Branford S, Soverini S, Kim DW, Muller MC et al. Impact of baseline BCR-ABL mutations on response to nilotinib in patients with chronic myeloid leukemia in chronic phase. J Clin Oncol 2009; 27: 4204–4210.
Jabbour E, Kantarjian H, Jones D, Talpaz M, Bekele N, O'Brien S et al. Frequency and clinical significance of BCR-ABL mutations in patients with chronic myeloid leukemia treated with imatinib mesylate. Leukemia 2006; 20: 1767–1773.
Muller MC, Cortes JE, Kim DW, Druker BJ, Erben P, Pasquini R et al. Dasatinib treatment of chronic-phase chronic myeloid leukemia: analysis of responses according to preexisting BCR-ABL mutations. Blood 2009; 114: 4944–4953.
Barnes DJ, Palaiologou D, Panousopoulou E, Schultheis B, Yong AS, Wong A et al. Bcr-Abl expression levels determine the rate of development of resistance to imatinib mesylate in chronic myeloid leukemia. Cancer Res 2005; 65: 8912–8919.
Campbell LJ, Patsouris C, Rayeroux KC, Somana K, Januszewicz EH, Szer J . BCR/ABL amplification in chronic myelocytic leukemia blast crisis following imatinib mesylate administration. Cancer Genet Cytogenet 2002; 139: 30–33.
De Braekeleer E, Douet-Guilbert N, Le Bris MJ, Morel F, De Braekeleer M . Translocation 3;21, trisomy 8, and duplication of the Philadelphia chromosome: a rare but recurrent cytogenetic pathway in the blastic phase of chronic myeloid leukemia. Cancer Genet Cytogenet 2007; 179: 159–161.
Gorre ME, Mohammed M, Ellwood K, Hsu N, Paquette R, Rao PN et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science 2001; 293: 876–880.
Hochhaus A, Kreil S, Corbin AS, La Rosee P, Muller MC, Lahaye T et al. Molecular and chromosomal mechanisms of resistance to imatinib (STI571) therapy. Leukemia 2002; 16: 2190–2196.
Donato NJ, Wu JY, Stapley J, Gallick G, Lin H, Arlinghaus R et al. BCR-ABL independence and LYN kinase overexpression in chronic myelogenous leukemia cells selected for resistance to STI571. Blood 2003; 101: 690–698.
Hayette S, Chabane K, Michallet M, Michallat E, Cony-Makhoul P, Salesse S et al. Longitudinal studies of SRC family kinases in imatinib- and dasatinib-resistant chronic myelogenous leukemia patients. Leuk Res 2011; 35: 38–43.
Mahon FX, Hayette S, Lagarde V, Belloc F, Turcq B, Nicolini F et al. Evidence that resistance to nilotinib may be due to BCR-ABL, Pgp, or Src kinase overexpression. Cancer Res 2008; 68: 9809–9816.
Wu J, Meng F, Kong LY, Peng Z, Ying Y, Bornmann WG et al. Association between imatinib-resistant BCR-ABL mutation-negative leukemia and persistent activation of LYN kinase. J Natl Cancer Inst 2008; 100: 926–939.
Wu J, Meng F, Lu H, Kong L, Bornmann W, Peng Z et al. Lyn regulates BCR-ABL and Gab2 tyrosine phosphorylation and c-Cbl protein stability in imatinib-resistant chronic myelogenous leukemia cells. Blood 2008; 111: 3821–3829.
Crossman LC, Druker BJ, Deininger MW, Pirmohamed M, Wang L, Clark RE . hOCT 1 and resistance to imatinib. Blood 2005; 106: 1133–1134; author reply 1134.
Wang L, Giannoudis A, Lane S, Williamson P, Pirmohamed M, Clark RE . Expression of the uptake drug transporter hOCT1 is an important clinical determinant of the response to imatinib in chronic myeloid leukemia. Clin Pharmacol Ther 2008; 83: 258–264.
White DL, Dang P, Engler J, Frede A, Zrim S, Osborn M et al. Functional activity of the OCT-1 protein is predictive of long-term outcome in patients with chronic-phase chronic myeloid leukemia treated with imatinib. J Clin Oncol 2010; 28: 2761–2767.
White DL, Radich J, Soverini S, Saunders VA, Frede A, Dang P et al. Chronic phase chronic myeloid leukemia patients with low OCT-1 activity randomised to high-dose imatinib achieve better responses, and lower failure rates, than those randomized to standard-dose. Haematologica 2012; 97: 907–914.
White DL, Saunders VA, Dang P, Engler J, Venables A, Zrim S et al. Most CML patients who have a suboptimal response to imatinib have low OCT-1 activity: higher doses of imatinib may overcome the negative impact of low OCT-1 activity. Blood 2007; 110: 4064–4072.
White DL, Saunders VA, Dang P, Engler J, Zannettino AC, Cambareri AC et al. OCT-1-mediated influx is a key determinant of the intracellular uptake of imatinib but not nilotinib (AMN107): reduced OCT-1 activity is the cause of low in vitro sensitivity to imatinib. Blood 2006; 108: 697–704.
Hiwase DK, Saunders V, Hewett D, Frede A, Zrim S, Dang P et al. Dasatinib cellular uptake and efflux in chronic myeloid leukemia cells: therapeutic implications. Clin Cancer Res 2008; 14: 3881–3888.
Eadie LN, Hughes TP, White DL . Interaction of the efflux transporters ABCB1 and ABCG2 with imatinib, nilotinib, and dasatinib. Clin Pharmacol Ther 2014; 95: 294–306.
Burger H, van Tol H, Brok M, Wiemer EA, de Bruijn EA, Guetens G et al. Chronic imatinib mesylate exposure leads to reduced intracellular drug accumulation by induction of the ABCG2 (BCRP) and ABCB1 (MDR1) drug transport pumps. Cancer Biol Ther 2005; 4: 747–752.
Gromicho M, Dinis J, Magalhaes M, Fernandes AR, Tavares P, Laires A et al. Development of imatinib and dasatinib resistance: dynamics of expression of drug transporters ABCB1, ABCC1, ABCG2, MVP, and SLC22A1. Leuk Lymphoma 2011; 52: 1980–1990.
Hirayama C, Watanabe H, Nakashima R, Nanbu T, Hamada A, Kuniyasu A et al. Constitutive overexpression of P-glycoprotein, rather than breast cancer resistance protein or organic cation transporter 1, contributes to acquisition of imatinib-resistance in K562 cells. Pharm Res 2008; 25: 827–835.
Illmer T, Schaich M, Platzbecker U, Freiberg-Richter J, Oelschlagel U, von Bonin M et al. P-glycoprotein-mediated drug efflux is a resistance mechanism of chronic myelogenous leukemia cells to treatment with imatinib mesylate. Leukemia 2004; 18: 401–408.
Mahon FX, Belloc F, Lagarde V, Chollet C, Moreau-Gaudry F, Reiffers J et al. MDR1 gene overexpression confers resistance to imatinib mesylate in leukemia cell line models. Blood 2003; 101: 2368–2373.
Mahon FX, Deininger MW, Schultheis B, Chabrol J, Reiffers J, Goldman JM et al. Selection and characterization of BCR-ABL positive cell lines with differential sensitivity to the tyrosine kinase inhibitor STI571: diverse mechanisms of resistance. Blood 2000; 96: 1070–1079.
Tang C, Schafranek L, Watkins DB, Parker WT, Moore S, Prime JA et al. Tyrosine kinase inhibitor resistance in chronic myeloid leukemia cell lines: investigating resistance pathways. Leuk Lymphoma 2011; 52: 2139–2147.
Widmer N, Colombo S, Buclin T, Decosterd LA . Functional consequence of MDR1 expression on imatinib intracellular concentrations. Blood 2003; 102: 1142.
White D, Saunders V, Grigg A, Arthur C, Filshie R, Leahy MF et al. Measurement of in vivo BCR-ABL kinase inhibition to monitor imatinib-induced target blockade and predict response in chronic myeloid leukemia. J Clin Oncol 2007; 25: 4445–4451.
White D, Saunders V, Lyons AB, Branford S, Grigg A, To LB et al. In vitro sensitivity to imatinib-induced inhibition of ABL kinase activity is predictive of molecular response in patients with de novo CML. Blood 2005; 106: 2520–2526.
Eadie LN, Saunders VA, Hughes TP, White DL . Degree of kinase inhibition achieved in vitro by imatinib and nilotinib is decreased by high levels of ABCB1 but not ABCG2. Leuk Lymphoma 2013; 54: 569–578.
Yeung DT, Osborn MP, White DL, Branford S, Braley J, Herschtal A et al. TIDEL-II: first-line use of imatinib in CML with early switch to nilotinib for failure to achieve time-dependent molecular targets. Blood 2015; 125: 915–923.
Chomczynski P, Sacchi N . Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal Biochem 1987; 162: 156–159.
Branford S, Fletcher L, Cross NC, Muller MC, Hochhaus A, Kim DW et al. Desirable performance characteristics for BCR-ABL measurement on an international reporting scale to allow consistent interpretation of individual patient response and comparison of response rates between clinical trials. Blood 2008; 112: 3330–3338.
Cross NC, Hughes TP, Hochhaus A, Goldman JM . International standardisation of quantitative real-time RT-PCR for BCR-ABL. Leuk Res 2008; 32: 505–506.
Branford S, Hughes TP, Rudzki Z . Monitoring chronic myeloid leukaemia therapy by real-time quantitative PCR in blood is a reliable alternative to bone marrow cytogenetics. Br J Haematol 1999; 107: 587–599.
Agrawal M, Hanfstein B, Erben P, Wolf D, Ernst T, Fabarius A et al. MDR1 expression predicts outcome of Ph+ chronic phase CML patients on second-line nilotinib therapy after imatinib failure. Leukemia 2014; 28: 1478–1485.
Giannoudis A, Davies A, Harris RJ, Lucas CM, Pirmohamed M, Clark RE . The clinical significance of ABCC3 as an imatinib transporter in chronic myeloid leukaemia. Leukemia 2014; 28: 1360–1363.
Kim YK, Lee SS, Jeong SH, Ahn JS, Yang DH, Lee JJ et al. OCT-1, ABCB1, and ABCG2 expression in imatinib-resistant chronic myeloid leukemia treated with dasatinib or nilotinib. Chonnam Med J 2014; 50: 102–111.
Branford S, Yeung DT, Parker WT, Roberts ND, Purins L, Braley JA et al. Prognosis for patients with CML and >10% BCR-ABL1 after 3 months of imatinib depends on the rate of BCR-ABL1 decline. Blood 2014; 124: 511–518.
Larson RA, Druker BJ, Guilhot F, O'Brien SG, Riviere GJ, Krahnke T et al. Imatinib pharmacokinetics and its correlation with response and safety in chronic-phase chronic myeloid leukemia: a subanalysis of the IRIS study. Blood 2008; 111: 4022–4028.
Picard S, Titier K, Etienne G, Teilhet E, Ducint D, Bernard MA et al. Trough imatinib plasma levels are associated with both cytogenetic and molecular responses to standard-dose imatinib in chronic myeloid leukemia. Blood 2007; 109: 3496–3499.
White D, Dang P, Venables A, Saunders V, Zrim S, Zannettino A et al. ABCB1 overexpression may predispose imatinib treated CML patients to the development of Abl kinase domain mutations, and may be an important contributor to acquired resistance. ASH Annu Meet Abstr 2006; 108: 2144.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
LNE, PD, VAS, MPO and APG have no conflict of interest to declare. DTY, TPH and DLW receive honoraria and research funds from Novartis Pharmaceuticals, and are members of Advisory Boards for Novartis. DTY and TPH are chairs of the CML/MPN disease group for the Australasian Leukaemia and Lymphoma Group (ALLG). Neither Novartis nor ALLG had roles in the design of the study, collection and analysis of the data or the decision to publish.
Additional information
Author contributions
LNE designed the experiments, performed the experiments, analysed the data, wrote the manuscript and created the figures. PD performed the experiments and reviewed the manuscript. VAS coordinated patient samples and reviewed the manuscript. DTY and MPO supervised conduct of TIDEL II, contributed patients and reviewed the manuscript. APG designed TIDEL II, supervised conduct of TIDEL II, contributed patients, served on the TIDEL II management committee and reviewed the paper. TPH designed the experiments, designed TIDEL II, supervised conduct of TIDEL II, contributed patients, served on the TIDEL II management committee and reviewed the paper. DLW designed the experiments, analysed the data, created the figures and reviewed the manuscript.
Supplementary Information accompanies this paper on the Leukemia website
Rights and permissions
About this article
Cite this article
Eadie, L., Dang, P., Saunders, V. et al. The clinical significance of ABCB1 overexpression in predicting outcome of CML patients undergoing first-line imatinib treatment. Leukemia 31, 75–82 (2017). https://doi.org/10.1038/leu.2016.179
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2016.179
This article is cited by
-
The importance of personalized medicine in chronic myeloid leukemia management: a narrative review
Egyptian Journal of Medical Human Genetics (2023)
-
Adverse outcomes for chronic myeloid leukemia patients with splenomegaly and low in vivo kinase inhibition on imatinib
Blood Cancer Journal (2023)
-
Rotating between ponatinib and imatinib temporarily increases the efficacy of imatinib as shown in a chronic myeloid leukaemia model
Scientific Reports (2022)
-
Long non-coding RNAs as the critical regulators of doxorubicin resistance in tumor cells
Cellular & Molecular Biology Letters (2021)
-
Why chronic myeloid leukaemia cannot be cured by tyrosine kinase-inhibitors
Leukemia (2021)