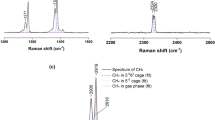
Abstract
Natural gas hydrates are solid, non-stoichiometric compounds of small gas molecules and water. They form when the constituents come into contact at low temperature and high pressure. The physical properties of these compounds, most notably that they are non-flowing crystalline solids that are denser than typical fluid hydrocarbons and that the gas molecules they contain are effectively compressed, give rise to numerous applications in the broad areas of energy and climate effects. In particular, they have an important bearing on flow assurance and safety issues in oil and gas pipelines, they offer a largely unexploited means of energy recovery and transportation, and they could play a significant role in past and future climate change.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Koen, B. V. Discussion of the Method: Conducting the Engineer's Approach to Problem Solving (Oxford Univ. Press, Oxford, 2003).
Sloan, E. D. in Clathrate Hydrates of Natural Gases (Marcel Dekker, New York, 1998).
Ripmeester, J. A. Hydrate research – from correlations to a knowledge-based discipline: the importance of structure. Ann. NY Acad Sci. 912, 1–16 (2000).
Sloan, E. D. in Hydrate Engineering (Soc. Petrol. Eng., Richardson, TX, 2000).
Makogon, Y. F. Hydrates of Hydrocarbons (Pennwell Books, Tulsa, 1997).
Sloan, E. D. Clathrate hydrate measurements: microscopic, mesoscopic, and macroscopic. J. Chem. Thermo. 35, 41–53 (2003).
Davidson, D. W. in Water: A Comprehensive Treatise (ed. Franks, F.) (Plenum, New York, 1973).
Englezos, P. Clathrate hydrates. Ind. Eng. Chem. Res. 32, 1251–1274 (1993).
Mao, W. L. et al. Hydrogen clusters in clathrate dydrate. Science 297, 2247–2249 (2002).
von Stackelberg, M. Solid gas hydrates. Naturwissenschaften 11-12, 1–22 (1949).
Huo, Z., Hester, K., Miller, K. T. & Sloan, E. D. Methane hydrate non-stoichiometry and phase diagram. Am. Ind. Chem. Eng. J. 49, 1300–1306 (2003).
Kobayashi, R. & Katz, D. L. Methane hydrate at high pressure. J. Petrol Technol. 2579, 66–70 (1949).
Holder, G. D. & Manganiello, D. J. Hydrate dissociation pressure minima in multicomponent systems. Chem. Eng. Sci. 37, 9–16 (1982).
Hendriks, E. M., Edmonds, B., Moorwood, R. A. & Szczepanski, R. Hydrate structure stability in simple and mixed dydrates. Fluid Phase Equilibria 117, 293–298 (1996).
Subramanian, S., Kini, R. A., Dec, S. F. & Sloan, E. D. Evidence of structure II hydrate formation from methane+ethane mixtures. Chem. Eng. Sci. 55, 1981–1985 (2000).
Staykova, D. K., Hansen, T., Salamatin, A. N. & Kuhs, W. F. Kinetic diffraction experiments on the formation of porous gas hydrates. Proc. 4th Int.Conf. Gas Hydrates (2002).
Hester, K. & Sloan, E. D. Structure II hydrates from binary structure I simple guests. Fluid Phase Equilibria (in the press).
Davidson, D. W., Handa, Y. P., Ratcliffe, C. I., Tse, J. S. & Powell, B. M. The ability of small molecules to form clathrate hydrates of structure II. Nature 311, 142–143 (1984).
Ripmeester, J. A., Ratcliffe, C. I. & Powell, B. M. A new clathrate hydrate structure. Nature 325, 135–136 (1987).
Ballard, A. & Sloan, E. D. The next generation of hydrate prediction: An overview. Proc. 4th Int.Conf. Gas Hydrates (2002).
van der Waals, J. H. & Platteeuw, J. C. Clathrate solutions. Adv. Chem. Phys. 2, 1–58 (1959).
Mehta, A. P., Hebert, P. B., Cadena, E. R. & Weatherman, J. P. Fulfilling the promise of low-dosage hydrate inhibitors: Journey from academic curiosity to successful field implementation. SPE Prod. Facil. 73–79 (2003).
Kvenvolden, K A. in Methane Hydrates: Resources in the near Future? (Proc. Int. Japan Natl Oil Comp, Chiba City, Japan, 1998)
Milkov, A. V. & Sassen, R. Resource and economic potential of gas hydrates in the northwestern gulf of Mexico. Proc. 4th Int.Conf. Gas Hydrates 111–114 (2002).
Soloviev, V., Ginsburg, G., Telepnevm, E. & Mikhailyk, Y. Cryogeothermy and Natural Gas Hydrates of the Arctic Ocean Sediments (Ministry of Geology USSR, Leningrad, 1987).
Dallimore, S. R. et al. (eds). Scientific Results from the Mallik 2002 Gas Hydrate Production Research Well Program, Mackenzie Delta, Northwest Territories, Canada. Bull. Geol. Soc. Can. 585 (in the press).
Dallimore, S. R., Collett, T. S. & Uchida, T. Summary of Mallik 21–38 Well GSC Bull. 544 1, 1–10 (1999).
Paull, C. K. & Matsumoto. R. Leg 164 overview. Proc. ODP Sci. Res. 164, 3–10 (2000).
Moridis, G. et al. Numerical simulation studies of gas production scenarios from hydrate accumulations at the Mallik Site, Mackenzie Delta, Canada. Proc. 4th Int.Conf. Gas Hydrates 239–244 (2002).
Gudmundsson, J. & Borrehaug, A. Frozen hydrate for transport of natural gas in Proc. 2nd Int. Conf. on Natural Gas Hydrates 415–422 (1996).
Nakajima, Y., Takaoki, T., Ohgaki, K. & Ota, S. Use of hydrate pellets for transportation of natural gas – II; Proposition of natural gas transportation in form of hydrate pellets in Proc. 4th Int.Conf. Gas Hydrates 987–990 (2002).
Shirota, H. et al. Measurement of methane hydrate dissociation for application to natural gas storage and transportation. Proc. 4th Int.Conf. Gas Hydrates (2002).
Gudmundsson, J., Andersson, V., Levik, O. I., Mork, M. & Borrehaug, A. Hydrate technology for capturing stranded gas. Ann. NY Acad. Science 912, 403–410.
Kennett, J. P., Cannariato, G., Hendy, I. L. & Behl, R. J. Methane Hydrates in Quaternary Climate Change: The Clathrate Gun Hypothesis (Am. Geophys. Union, Washington DC, 2003).
Dickens, G. R., Castillo, M. M. & Walker, J. C. G. A blast of gas in the latest paleocene: simulating first-order effects of massive dissociation of ocean methane hydrate. Geology 25, 259–262 (1997).
Dickens, G., O'Neil, J., Rea, D. & Owen, R. Dissociation of oceanic methane hydrates as a cause of the carbon isotope excursion at the end of the Palaeocene. Paleoceanography 10, 965–971 (1995).
Kaiho, K. et al. Latest palaeocene benthic foramiferal extinction and environmental changes at Tawanui, New Zealand. Paleoceanography 11, 447–465 (1996).
Maslin, M. & Thomas, E. The clathrate gun is firing blanks: Evidence from balancing the deglacial global carbon budget. Geophys. Res. Abstr. 5, 12015 (2003).
Dickens, G. R. A methane trigger for rapid warming? Science 299, 1017 (2003).
Dillon, W. P et al. in Gas Hydrates: Relevance to World Margin Stability and Climate Change (eds Henriet, J.-P & Mienert, J.) 293–302 (Geol. Soc., London, 1998).
Paull, C. K. & Dillon, W. P. (eds) Natural Gas Hydrates: Occurrence, Distribution and Detection (Am. Geophys. Union, Washington DC, 2001).
Bishoi, P. R. & Natarajan, V. Formation and decomposition of gas hydrates. Fluid Phase Equilibria 117, 168–177 (1996).
Sloan, E. D. Jr in Gas Hydrates: Relevance to World Margin Stability and Climate Change (eds Henriet, J.-P & Mienart, J.) 31–40 (Spec. Publ. 137, Geol. Soc, London, 1998).
Dickens, G. R. Methane oxidation during the Late Palaeocene Thermal Maximum. Bull. Soc. Geol. Fr. 171, 37–49 (2000).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sloan, E. Fundamental principles and applications of natural gas hydrates. Nature 426, 353–359 (2003). https://doi.org/10.1038/nature02135
Issue Date:
DOI: https://doi.org/10.1038/nature02135
This article is cited by
-
Empirical evaluation of the strength and deformation characteristics of natural and synthetic gas hydrate-bearing sediments with different ranges of porosity, hydrate saturation, effective stress, and strain rate
Progress in Earth and Planetary Science (2024)
-
Probing the critical nucleus size in tetrahydrofuran clathrate hydrate formation using surface-anchored nanoparticles
Nature Communications (2024)
-
Numerical simulation of hydraulic fracture characteristics in gas hydrate bearing-sediments by using CDEM
Acta Mechanica Sinica (2024)
-
Quantitative study of drilling-induced core damage through laboratory tests
Acta Geotechnica (2024)
-
Rietveld Analysis of Binary (2,5-Dihydrofuran + Methane) and (2,3-Dihydrofuran + Methane) Clathrate Hydrates
Korean Journal of Chemical Engineering (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.