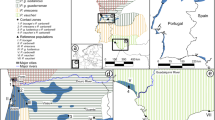
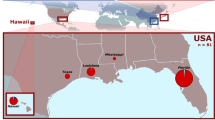
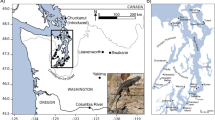
Abstract
A genetic paradox1,2 exists in invasion biology: how do introduced populations, whose genetic variation has probably been depleted by population bottlenecks, persist and adapt to new conditions? Lessons from conservation genetics show that reduced genetic variation due to genetic drift and founder effects limits the ability of a population to adapt, and small population size increases the risk of extinction1,3,4. Nonetheless, many introduced species experiencing these same conditions during initial introductions persist, expand their ranges, evolve rapidly and become invasive. To address this issue, we studied the brown anole, a worldwide invasive lizard. Genetic analyses indicate that at least eight introductions have occurred in Florida from across this lizard's native range, blending genetic variation from different geographic source populations and producing populations that contain substantially more, not less, genetic variation than native populations. Moreover, recently introduced brown anole populations around the world originate from Florida, and some have maintained these elevated levels of genetic variation. Here we show that one key to invasion success may be the occurrence of multiple introductions that transform among-population variation in native ranges to within-population variation in introduced areas. Furthermore, these genetically variable populations may be particularly potent sources for introductions elsewhere. The growing problem of invasive species introductions brings considerable economic and biological costs5,6. If these costs are to be mitigated, a greater understanding of the causes, progression and consequences of biological invasions is needed7.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Allendorf, F. W. & Lundquist, L. L. Introduction: population biology, evolution, and control of invasive species. Conserv. Biol. 17, 24–30 (2003)
Sakai, A. K. et al. The population biology of invasive species. Annu. Rev. Ecol. Syst. 32, 305–332 (2001)
Frankham, R., Ballou, J. D. & Briscoe, D. A. Introduction to Conservation Genetics (Cambridge Univ. Press, Cambridge, 2002)
Frankham, R. & Ralls, K. Conservation biology: inbreeding leads to extinction. Nature 392, 441–442 (1998)
Pimentel, D., Lach, L., Zuniga, R. & Morrison, D. Environmental and economic costs of nonindigenous species in the United States. Bioscience 50, 53–65 (2000)
Wilcove, D. S., Rothstein, D., Dubow, J., Phillips, A. & Losos, E. Quantifying threats to imperiled species in the United States. Bioscience 48, 607–615 (1998)
Mack, R. N. et al. Biotic invasions: causes, epidemiology, global consequences, and control. Ecol. Appl. 10, 689–710 (2000)
Kolar, C. S. & Lodge, D. M. Ecological predictions and risk assessment for alien fishes in North America. Science 298, 1233–1235 (2002)
Lee, C. E. Evolutionary genetics of invasive species. Trends Ecol. Evol. 17, 386–391 (2002)
Queller, D. C. Pax Argentinica. Nature 405, 519–520 (2000)
Huey, R. B., Gilchrist, G. W., Carlson, M. L., Berrigan, D. B. & Serra, L. Rapid evolution of a geographic cline in size in an introduced population. Science 287, 308–309 (2000)
Losos, J. B., Marks, J. C. & Schoener, T. W. Habitat use and ecological interactions of an introduced and a native species of Anolis lizard on Grand Cayman, with a review of the outcomes of anole introductions. Oecologia 95, 525–532 (1993)
Campbell, T. S. Analysis of the effects of an exotic lizard (Anolis sagrei) on a native lizard (Anolis carolinensis) in Florida, using islands as experimental units. PhD thesis, Univ. Tennessee (2000)
Gerber, G. P. & Echternacht, A. C. Evidence for asymmetrical intraguild predation between native and introduced Anolis lizards. Oecologia 124, 599–607 (2000)
Williams, E. E. The ecology of colonization as seen in the zoogeography of anoline lizards on small islands. Q. Rev. Biol. 44, 345–389 (1969)
Campbell, T. S. Northern range expansion of the brown anole (Anolis sagrei) in Florida and Georgia. Herpetol. Rev. 27, 155–157 (1996)
Lee, J. C. Anolis sagrei in Florida: phenetics of a colonizing species I. Meristic characters. Copeia 1985, 182–194 (1985)
Novak, S. J. & Mack, R. N. Genetic variation in Bromus tectorum (Poaceae): comparison between native and introduced populations. Heredity 71, 167–176 (1993)
Facon, B. et al. A molecular phylogeography approach to biological invasions of the New World by parthenogenetic Thiarid snails. Mol. Ecol. 12, 3027–3039 (2003)
Williamson, M. Biological Invasions (Chapman & Hall, London, 1996)
Tsutsui, N. D., Suarez, A. V., Holway, D. A. & Case, T. J. Reduced genetic variation and the success of an invasive species. Proc. Natl Acad. Sci. USA 97, 5948–5953 (2000)
Lee, J. C. Anolis sagrei in Florida: phenetics of a colonizing species III. West Indian and Middle American comparisons. Copeia 1992, 942–954 (1992)
Campbell, T. S. & Echternacht, A. C. Introduced species as moving targets: changes in body sizes of introduced lizards following experimental introductions and historical invasions. Biol. Invasions 5, 193–212 (2003)
Ellstrand, N. C. & Schierenbeck, K. A. Hybridization as a stimulus for the evolution of invasiveness in plants? Proc. Natl Acad. Sci. USA 97, 7043–7050 (2000)
Mooney, H. A. & Cleland, E. E. The evolutionary impact of invasive species. Proc. Natl Acad. Sci. USA 98, 5446–5451 (2001)
Ehrlich, P. R. in Biological Invasions: A Global Perspective (eds Drake, J. A. et al.) 315–328 (Wiley, Chichester, 1989)
Roderick, G. K. & Navajas, M. Genes in new environments: genetics and evolution in biological control. Nature Rev. Genet. 4, 889–899 (2003)
Macey, J. R., Larson, A., Ananjeva, N. B. & Papenfuss, T. J. Evolutionary shifts in three major structural features of the mitochondrial genome among iguanian lizards. J. Mol. Evol. 44, 660–674 (1997)
Huelsenbeck, J. P. & Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17, 754–755 (2001)
Nylander, J.A.A. MrModeltest v1.0b (Department of Systematic Zoology, Uppsala University, 2002).
Acknowledgements
We thank T. Campbell, D. Cardace, P. Colbert, K. de Queiroz, A. Echternacht, J. Gaskin, L. Harmon, J. Knouft, K. Kozak, R. Muller, G. Norval, S. Poe, R. Powell, V. Rivalta Gonzalez, A. Torres Barboza and A. Wright for advice and assistance, and the National Science Foundation and the Environmental Protection Agency Science to Achieve Results (STAR) program for funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Supplementary Information
The SI file contains 3 figures with legends, 3 tables with legends, and associated references and notes. (DOC 633 kb)
Rights and permissions
About this article
Cite this article
Kolbe, J., Glor, R., Rodríguez Schettino, L. et al. Genetic variation increases during biological invasion by a Cuban lizard. Nature 431, 177–181 (2004). https://doi.org/10.1038/nature02807
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature02807
This article is cited by
-
Complex European invasion history of Anoplophora glabripennis (Motschulsky): new insights in its population genomic differentiation using genotype-by-sequencing
Scientific Reports (2024)
-
Population genetic structure of invasive apple snails Pomacea maculata in Louisiana
Aquatic Ecology (2024)
-
Genetic variation of European mouflon depends on admixture of introduced individuals
Mammal Research (2024)
-
On the brink of explosion? Identifying the source and potential spread of introduced Zosterops white-eyes in North America
Biological Invasions (2024)
-
Population mixing mediates the intestinal flora composition and facilitates invasiveness in a globally invasive fruit fly
Microbiome (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.