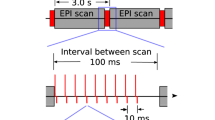
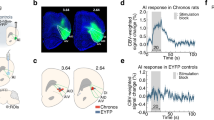
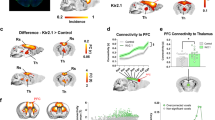
Abstract
Despite a rapidly-growing scientific and clinical brain imaging literature based on functional magnetic resonance imaging (fMRI) using blood oxygenation level-dependent (BOLD)1 signals, it remains controversial whether BOLD signals in a particular region can be caused by activation of local excitatory neurons2. This difficult question is central to the interpretation and utility of BOLD, with major significance for fMRI studies in basic research and clinical applications3. Using a novel integrated technology unifying optogenetic4,5,6,7,8,9,10,11,12,13 control of inputs with high-field fMRI signal readouts, we show here that specific stimulation of local CaMKIIα-expressing excitatory neurons, either in the neocortex or thalamus, elicits positive BOLD signals at the stimulus location with classical kinetics. We also show that optogenetic fMRI (ofMRI) allows visualization of the causal effects of specific cell types defined not only by genetic identity and cell body location, but also by axonal projection target. Finally, we show that ofMRI within the living and intact mammalian brain reveals BOLD signals in downstream targets distant from the stimulus, indicating that this approach can be used to map the global effects of controlling a local cell population. In this respect, unlike both conventional fMRI studies based on correlations14 and fMRI with electrical stimulation that will also directly drive afferent and nearby axons, this ofMRI approach provides causal information about the global circuits recruited by defined local neuronal activity patterns. Together these findings provide an empirical foundation for the widely-used fMRI BOLD signal, and the features of ofMRI define a potent tool that may be suitable for functional circuit analysis as well as global phenotyping of dysfunctional circuitry.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
10 June 2010
Minor corrections were made to Fig. 1d and to the text of the paragraph beginning, 'To assess fMRI signals ...'
References
Ogawa, S. et al. Intrinsic signal changes accompanying sensory stimulation: Functional brain mapping with magnetic resonance imaging. Proc. Natl Acad. Sci. USA 89, 5951–5955 (1992)
Logothetis, N. K., Pauls, J., Augath, M., Trinath, T. & Oeltermann, A. Neurophysiological investigation of the basis of the fMRI signal. Nature 412, 150–157 (2001)
Cohen, J. D. & Blum, K. I. Reward and decision. Neuron 36, 193–198 (2002)
Boyden, E. S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nature Neurosci. 8, 1263–1268 (2005)
Zhang, F., Wang, L. P., Boyden, E. S. & Deisseroth, K. Channelrhodopsin-2 and optical control of excitable cells. Nature Methods 3, 785–792 (2006)
Deisseroth, K. et al. Next-generation optical technologies for illuminating genetically targeted brain circuits. J. Neurosci. 26, 10380–10386 (2006)
Zhang, F. et al. Multimodal fast optical interrogation of neural circuitry. Nature 446, 633–639 (2007)
Zhang, F., Aravanis, A. M., Adamantidis, A., de Lecea, L. & Deisseroth, K. Circuit-breakers: optical technologies for probing neural signals and systems. Nature Rev. Neurosci. 8, 577–581 (2007)
Aravanis, A. M. et al. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J. Neural Eng. 4, S143–S156 (2007)
Sohal, V. S., Zhang, F., Yizhar, O. & Deisseroth, K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459, 698–702 (2009)
Gradinaru, V., Mogri, M., Thompson, K. R., Henderson, J. M. & Deisseroth, K. Optical deconstruction of parkinsonian neural circuitry. Science 324, 354–359 (2009)
Zhang, F. et al. Optogenetic interrogation of neural circuits: technology for probing mammalian brain structures. Nature Protocols 5, 439–456 (2010)
Gradinaru, V. et al. Molecular and cellular approaches for diversifying and extending optogenetics. Cell 141, 154–165 (2010)
Friston, K. J. Functional and effective connectivity in neuroimaging: a synthesis. Hum. Brain Mapp. 2, 56–78 (1994)
Uğurbil, K. et al. Functional mapping in the human brain using high magnetic fields. Phil. Trans. R. Soc. Lond. B 354, 1195–1213 (1999)
Logothetis, N. K. What we can do and what we cannot do with fMRI. Nature 453, 869–878 (2008)
Douglas, R. J. & Martin, K. A. A functional microcircuit for cat visual cortex. J. Physiol. (Lond.) 440, 735–769 (1991)
Sirotin, Y. B. & Das, A. Anticipatory haemodynamic signals in sensory cortex not predicted by local neuronal activity. Nature 457, 475–479 (2009)
Cauli, B. et al. Cortical GABA Interneurons in neurovascular coupling: relays for subcortical vasoactive pathways. J. Neurosci. 24, 8940–8949 (2004)
Masamoto, K., Kim, T., Fukuda, M., Wang, P. & Kim, S. G. Relationship between neural, vascular, and BOLD signals in isoflurane-anesthetized rat somatosensory cortex. Cereb. Cortex 17, 942–950 (2007)
Lee, J. H. et al. Full-brain coverage and high-resolution imaging capabilities of passband b-SSFP fMRI at 3T. Magn. Reson. Med. 59, 1099–1110 (2008)
Lee, J. H., Hargreaves, B. A., Hu, B. S. & Nishimura, D. G. Fast 3D imaging using variable-density spiral trajectories with applications to limb perfusion. Magn. Reson. Med. 50, 1276–1285 (2003)
Glover, G. H. & Lee, A. T. Motion artifacts in fMRI: comparison of 2DFT with PR and spiral scan methods. Magn. Reson. Med. 33, 624–635 (1995)
Buxton, R. B., Wong, E. C. & Frank, L. R. Dynamics of blood flow and oxygenation changes during brain activation: the balloon model. Magn. Reson. Med. 39, 855–864 (1998)
Boynton, G. M., Engel, S. A., Glover, G. H. & Heeger, D. J. Linear systems analysis of functional magnetic resonance imaging in human V1. J. Neurosci. 16, 4207–4221 (1996)
Donahue, M. J. et al. Theoretical and experimental investigation of the VASO contrast mechanism. Magn. Reson. Med. 56, 1261–1273 (2006)
Lauritzen, M. Reading vascular changes in brain imaging: is dendritic calcium the key? Nature Rev. Neurosci. 6, 77–85 (2005)
Nir, Y., Dinstein, I., Malach, R. & Heeger, D. J. BOLD and spiking activity. Nature Neurosci. 11, 523–524, author reply 524 (2008)
Alloway, K. D., Olson, M. L. & Smith, J. B. Contralateral corticothalamic projections from MI whisker cortex: potential route for modulating hemispheric interactions. J. Comp. Neurol. 510, 100–116 (2008)
Kuramoto, E. et al. Two types of thalamocortical projections from the motor thalamic nuclei of the rat: a single neuron-tracing study using viral vectors. Cereb. Cortex 19, 2065–2077 (2009)
Acknowledgements
J.H.L. is supported by NIH pathway to independence grant (K99/R00) 1K99EB008738. R.D. is supported by an NSF Graduate Research Fellowship. V.G. is supported by SGF and SIGF (Stanford Graduate Fellowships). We acknowledge G. H. Glover, J. M. Pauly and D. G. Nishimura for generous support and advice, and C. Pacharinsak for assistance with rat intubation. We would also like to thank the entire Deisseroth laboratory for discussions and support, and Lee laboratory students, V. Karasev, Z. Fang and C. Jones for help with histological quantification and fMRI data analysis. K.D. is supported by the Keck, Snyder, Woo, Yu, McKnight, and Coulter Foundations, as well as by CIRM, NIMH, NIDA and the NIH Director’s Pioneer Award.
Author information
Authors and Affiliations
Contributions
J.H.L., F.Z., R.D., V.G. and K.D. designed the experiments. D.-S.K. provided information on the animal fMRI setup, J.H.L. developed the fMRI methods, and J.H.L. and R.D. conducted all ofMRI experiments. R.D., V.G. and F.Z. conducted animal surgery and preparation. J.H.L., L.E.F., R.D. and V.G. conducted optrode recordings. R.D., V.G. and I.G. acquired the confocal microscope images. J.H.L., R.D. and I.G. performed histology and confocal imaging for quantification. C.R. prepared the viral vectors. J.H.L., R.D., V.G., F.Z., D.-S.K. and K.D. prepared the figures and wrote the paper. K.D. supervised all aspects of the work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Methods, References and Supplementary Figures S1-S5 with legends. (PDF 5117 kb)
Rights and permissions
About this article
Cite this article
Lee, J., Durand, R., Gradinaru, V. et al. Global and local fMRI signals driven by neurons defined optogenetically by type and wiring. Nature 465, 788–792 (2010). https://doi.org/10.1038/nature09108
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature09108
This article is cited by
-
Synaptic-like transmission between neural axons and arteriolar smooth muscle cells drives cerebral neurovascular coupling
Nature Neuroscience (2024)
-
Functional neuroimaging as a catalyst for integrated neuroscience
Nature (2023)
-
Optogenetic stimulation of anterior insular cortex neurons in male rats reveals causal mechanisms underlying suppression of the default mode network by the salience network
Nature Communications (2023)
-
Spatio-temporal activation patterns of neuronal population evoked by optostimulation and the comparison to electrical microstimulation
Scientific Reports (2023)
-
Functional MRI reveals brain-wide actions of thalamically-initiated oscillatory activities on associative memory consolidation
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.