Abstract
Nanometre-scale pores and capillaries have long been studied because of their importance in many natural phenomena and their use in numerous applications1. A more recent development is the ability to fabricate artificial capillaries with nanometre dimensions, which has enabled new research on molecular transport and led to the emergence of nanofluidics2,3,4. But surface roughness in particular makes it challenging to produce capillaries with precisely controlled dimensions at this spatial scale. Here we report the fabrication of narrow and smooth capillaries through van der Waals assembly5, with atomically flat sheets at the top and bottom separated by spacers made of two-dimensional crystals6 with a precisely controlled number of layers. We use graphene and its multilayers as archetypal two-dimensional materials to demonstrate this technology, which produces structures that can be viewed as if individual atomic planes had been removed from a bulk crystal to leave behind flat voids of a height chosen with atomic-scale precision. Water transport through the channels, ranging in height from one to several dozen atomic planes, is characterized by unexpectedly fast flow (up to 1 metre per second) that we attribute to high capillary pressures (about 1,000 bar) and large slip lengths. For channels that accommodate only a few layers of water, the flow exhibits a marked enhancement that we associate with an increased structural order in nanoconfined water. Our work opens up an avenue to making capillaries and cavities with sizes tunable to ångström precision, and with permeation properties further controlled through a wide choice of atomically flat materials available for channel walls.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Israelachvili, J. N. Intermolecular and Surface Forces 3rd edn (Academic, 2011)
Eijkel, J. T. & van den Berg, A. Nanofluidics: what is it and what can we expect from it? Microfluid . Nanofluidics 1, 249–267 (2005)
Schoch, R. B., Han, J. & Renaud, P. Transport phenomena in nanofluidics. Rev. Mod. Phys. 80, 839–883 (2008)
Howorka, S. & Siwy, Z. Nanopore analytics: sensing of single molecules. Chem. Soc. Rev. 38, 2360–2384 (2009)
Geim, A. K. & Grigorieva, I. V. Van der Waals heterostructures. Nature 499, 419–425 (2013)
Novoselov, K. S. et al. Two-dimensional atomic crystals. Proc. Natl Acad. Sci. USA 102, 10451–10453 (2005)
Mijatovic, D., Eijkel, J. C. T. & van den Berg, A. Technologies for nanofluidic systems: top-down vs. bottom-up — a review. Lab Chip 5, 492–500 (2005)
Duan, C. & Majumdar, A. Anomalous ion transport in 2-nm hydrophilic nanochannels. Nat. Nanotechnol. 5, 848–852 (2010)
Duan, C., Wang, W. & Xie, Q. Review article: fabrication of nanofluidic devices. Biomicrofluidics 7, 026501 (2013)
Hinds, B. J. et al. Aligned multiwalled carbon nanotube membranes. Science 303, 62–65 (2004)
Majumder, M., Chopra, N., Andrews, R. & Hinds, B. J. Nanoscale hydrodynamics: enhanced flow in carbon nanotubes. Nature 438, 44 (2005)
Holt, J. K. et al. Fast mass transport through sub-2-nanometer carbon nanotubes. Science 312, 1034–1037 (2006)
Thomas, J. A. & McGaughey, A. J. H. Reassessing fast water transport through carbon nanotubes. Nano Lett. 8, 2788–2793 (2008)
Falk, K., Sedlmeier, F., Joly, L., Netz, R. R. & Bocquet, L. Molecular origin of fast water transport in carbon nanotube membranes: superlubricity versus curvature dependent friction. Nano Lett. 10, 4067–4073 (2010)
Majumder, M., Chopra, N. & Hinds, B. J. Mass transport through carbon nanotube membranes in three different regimes: ionic diffusion and gas and liquid flow. ACS Nano 5, 3867–3877 (2011)
Qin, X., Yuan, Q., Zhao, Y., Xie, S. & Liu, Z. Measurement of the rate of water translocation through carbon nanotubes. Nano Lett. 11, 2173–2177 (2011)
Kannam, S. K., Todd, B. D., Hansen, J. S. & Daivis, P. J. How fast does water flow in carbon nanotubes? J. Chem. Phys. 138, 094701 (2013)
Garaj, S. et al. Graphene as a subnanometre trans-electrode membrane. Nature 467, 190–193 (2010)
Koenig, S. P., Wang, L., Pellegrino, J. & Bunch, J. S. Selective molecular sieving through porous graphene. Nat. Nanotechnol. 7, 728–732 (2012)
Celebi, K. et al. Ultimate permeation across atomically thin porous graphene. Science 344, 289–292 (2014)
O’Hern, S. C. et al. Nanofiltration across defect-sealed nanoporous monolayer graphene. Nano Lett. 15, 3254– 3260 (2015)
Wang, L. et al. Molecular valves for controlling gas phase transport made from discrete ångström-sized pores in graphene. Nat. Nanotechnol. 10, 785–790 (2015)
Jain, T. et al. Heterogeneous sub-continuum ionic transport in statistically isolated graphene nanopores. Nat. Nanotechnol. 10, 1053–1057 (2015)
Haigh, S. J. et al. Cross-sectional imaging of individual layers and buried interfaces of graphene-based heterostructures and superlattices. Nat. Mater. 11, 764–767 (2012)
Mücksch, C., Rösch, C., Müller-Renno, C., Ziegler, C. & Urbassek, H. M. Consequences of hydrocarbon contamination for wettability and protein adsorption on graphite surfaces. J. Phys. Chem. C 119, 12496–12501 (2015)
DasGupta, S., Schonberg, J. A., Kim, I. Y. & Wayner, P. C. Use of the augmented Young-Laplace equation to model equilibrium and evaporating extended menisci. J. Colloid Interface Sci. 157, 332–342 (1993)
Hummer, G., Rasaiah, J. C. & Noworyta, J. P. Water conduction through the hydrophobic channel of a carbon nanotube. Nature 414, 188–190 (2001)
Raviv, U., Laurat, P. & Klein, J. Fluidity of water confined to subnanometre films. Nature 413, 51–54 (2001)
Zhao, W.-H. et al. Highly confined water: two-dimensional ice, amorphous ice, and clathrate hydrates. Acc. Chem. Res. 47, 2505–2513 (2014)
Mate, C. M. Taking a fresh look at disjoining pressure of lubricants at slider-disk interfaces. IEEE Trans. Magn. 47, 124–130 (2011)
Gravelle, S., Ybert, C., Bocquet, L. & Joly, L. Anomalous capillary filling and wettability reversal in nanochannels. Phys. Rev. E 93, 033123 (2016)
Hu, S. et al. Proton transport through one-atom-thick crystals. Nature 516, 227–230 (2014)
Withers, F. et al. Light-emitting diodes by band-structure engineering in van der Waals heterostructures. Nat. Mater. 14, 301–306 (2015)
Schaffer, M., Schaffer, B. & Ramasse, Q. Sample preparation for atomic-resolution stem at low voltages by FIB. Ultramicroscopy 114, 62–71 (2012)
Livesey, R. G. Foundations of Vacuum Science and Technology (Wiley & Sons, 1998)
Stein, D., Kruithof, M. & Dekker, C. Surface-charge-governed ion transport in nanofluidic channels. Phys. Rev. Lett. 93, 035901 (2004)
Bocquet, L. & Charlaix, E. Nanofluidics, from bulk to interfaces. Chem. Soc. Rev. 39, 1073–1095 (2010)
Pang, P., He, J., Park, J. H., Krstic´, P. S. & Lindsay, S. Origin of giant ionic currents in carbon nanotube channels. ACS Nano 5, 7277–7283 (2011)
Cussler, E. L. Diffusion: Mass Transfer in Fluid Systems 3rd edn (Cambridge Univ. Press, 2009)
Berendsen, H. J. C., Grigera, J. R. & Straatsma, T. P. The missing term in effective pair potentials. J. Phys. Chem. 91, 6269–6271 (1987)
Hirunsit, P. & Balbuena, P. B. Effects of confinement on water structure and dynamics: a molecular simulation study. J. Phys. Chem. C 111, 1709–1715 (2007)
Koga, K. & Tanaka, H. Phase diagram of water between hydrophobic surfaces. J. Chem. Phys. 122, 104711 (2005)
Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 117, 1–19 (1995)
Kannam, K. S., Todd, B. D., Hansen, J. S. & Daivis, P. J. Slip length of water on graphene: limitations of non-equilibrium molecular dynamics simulations. J. Chem. Phys. 136, 024705 (2012)
Bocquet, L. & Barrat, J.-L. Flow boundary conditions from nano- to micro-scales. Soft Matter 3, 685–693 (2007)
Neek-Amal, M., Peeters, F. M., Grigorieva, I. V. & Geim, A. K. Commensurability effects in the viscosity of nanoconfined water. ACS Nano 10, 3685–3692 (2016)
Abascal, J. L. F. & Vega, C. A general purpose model for the condensed phases of water: TIP4P/2005. J. Chem. Phys. 123, 234505 (2005)
Werder, T., Walther, J. H., Jaffe, R. L., Halicioglu, T. & Koumoutsakos, P. On the water–carbon interaction for use in molecular dynamics simulations of graphite and carbon nanotubes. J. Phys. Chem. B 107, 1345–1352 (2003)
Kumar, P., Buldyrev, S. V., Starr, F. W., Giovambattista, N. & Stanley, H. E. Thermodynamics, structure, and dynamics of water confined between hydrophobic plates. Phys. Rev. E 72, 051503 (2005)
Mosaddeghi, H., Alavi, S., Kowsari, M. H. & Najafi, B. Simulations of structural and dynamic anisotropy in nano-confined water between parallel graphite plates. J. Chem. Phys. 137, 184703 (2012)
Lukas, M. et al. Catalytic subsurface etching of nanoscale channels in graphite. Nat. Commun. 4, 1379 (2013)
White, F. M. Viscous Fluid Flow 2nd edn (McGraw-Hill, 1991)
Li, J.-L. et al. Use of dielectric functions in the theory of dispersion forces. Phys. Rev. B 71, 235412 (2005)
Acknowledgements
This work was supported by the Lloyd’s Register Foundation, the European Research Council and the Royal Society. B.R. and K.G. acknowledge Marie Curie International Incoming Fellowships. S.J.H. and A.P.R. acknowledge the EPSRC NowNANO programme, EP/M022498/1, EP/K016946/1 and DTRA (HDTRA1-12-1-0013) for funding.
Author information
Authors and Affiliations
Contributions
A.K.G. and B.R. designed and directed the project. B.R. led development of the fabrication techniques. H.A.W. and F.C.W. provided theory support. B.R., A.E., K.G., A.J. and A.K. performed measurements and analysed results. A.P.R., S.J.H. and L.F. provided electron microscopy imaging. A.K.G., B.R., I.V.G., H.A.W. and F.C.W. wrote the manuscript. All authors contributed to discussions.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks L. Bocquet, C. Duan, J. Eijkel and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Extended data figures and tables
Extended Data Figure 1 Microfabrication process flow.
(1) A micrometre-scale hole is prepared in a silicon nitride membrane. (2) Bottom graphite is transferred to cover the opening. (3) An array of graphene spacers is transferred on top. (4) The hole is extended into the graphite–graphene stack by dry etching. (5) Top graphite crystal is transferred to cover the resulting aperture. The accompanying optical images (in natural colours) illustrate the results after each step for one of our devices. Graphene spacers are invisible in the photos and indicated by an opaque rectangle in (3). Steps 3 and 4 were often interchanged.
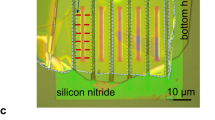
Extended Data Figure 2 Additional images of graphene capillaries.
a, Optical image of a final device. The green region is the free-standing silicon nitride membrane. The Si wafer is seen in brown and the top graphite crystal (arrowed) in yellow. Red, yellow and grey contours indicate positions of the top graphite, bottom graphite and graphene spacers, respectively. The nearly-vertical dark lines are wrinkles in the bottom layer. b, AFM image of four-layer graphene spacers on top of a bottom graphite crystal (height profile along the dashed line is shown below the image). Inset, high-resolution scan (friction mode) from the region indicated by the arrow. The observation of the atomic lattice confirms that our assemblies have atomically smooth surfaces. Such smoothness is impossible to achieve using conventional materials and processes that invariably lead to the surface roughness exceeding the scale given by few-layer graphene spacers. Although the side walls of our channels are rough due to limitations of electron-beam lithography, we estimate that, because of the large ratios w/h, the side wall contribution to the flow resistance cannot exceed 5% even for our 10 nm devices52. c, SEM micrograph of a capillary device with h ≈ 15 nm. d, Bright field STEM image of a graphene capillary with N = 4. Spacers and channels are arrowed in c and d.
Extended Data Figure 3 Sagging of top graphite.
a, Left, AFM image of trilayer channels, which are covered by a graphite layer of varying thickness. b, Left, partial sagging of the top graphite into wide channels. We can see that the top graphite bends down into the channels over their entire height h ≈ 5 nm. Right, height profiles that correspond to the traces shown by the dashed lines in the AFM images at left.
Extended Data Figure 4 He leak through graphene capillaries.
a, Schematic of our set-up. Two vacuum chambers are separated by the silicon nitride wafer incorporating a nanocapillary device. Valves connect the chambers to a pump, a He leak detector and a gas inlet. He atoms are represented by filled orange circles. b, Leak rates normalized for 1 μm length and given per channel as a function of applied pressure for capillary devices with N = 5 and N ≈ 45 (h ≈ 1.7 nm and 15 nm, respectively), and a control device without graphene spacers (N = 0).
Extended Data Figure 5 Ion transport through graphene nanochannels.
a, Schematic of our measurement set-up. A nanocapillary device fabricated on top of a Si nitride wafer (SiN is shown in green) is clamped using O-rings (black) to separate two containers (indicated by magenta lines). The containers are filled with a KCl solution (blue), and silver chloride–silver wires (dark grey) are used as electrodes to measure ionic conductance. b, Examples of current–voltage (I–V) characteristics of the smallest capillary devices (N = 2) at different KCl concentrations (labelling the curves; L ranges from 2.8 μm to 7 μm). c, Same as b but for a device with N ≈ 17 of approximately the same average length  (L from 1.7 μm to 7.3 μm). d, Ionic conductance for these devices as a function of KCl concentration, C (without normalizing for their slightly different
(L from 1.7 μm to 7.3 μm). d, Ionic conductance for these devices as a function of KCl concentration, C (without normalizing for their slightly different  ). Both blank Si nitride wafers separating the reservoirs and control devices with N = 0 (no spacers but otherwise prepared using the same fabrication procedures) exhibited leakage conductance of the order of 20 pS, which did not change with C (olive symbols). The dashed lines show ionic conductance G expected from the bulk conductivity of KCl for the given channel dimensions. The solid curves are fits taking into account an additional parallel conductance due to the surface charge.
). Both blank Si nitride wafers separating the reservoirs and control devices with N = 0 (no spacers but otherwise prepared using the same fabrication procedures) exhibited leakage conductance of the order of 20 pS, which did not change with C (olive symbols). The dashed lines show ionic conductance G expected from the bulk conductivity of KCl for the given channel dimensions. The solid curves are fits taking into account an additional parallel conductance due to the surface charge.
Extended Data Figure 6 Gravimetric measurements.
a, Extended schematic of the experimental set-up. A small aluminium container filled with water is sealed with a Si nitride wafer containing a graphene capillary device (total weight should not exceed ~15 g to allow the required measurement accuracy). The container was weighed either upside down (water in contact with capillaries as shown in the sketch) or in the upright position as shown in the inset of Fig. 2a (capillaries are exposed to 100% RH). Both orientations resulted in the same Q. b, Photographs of our gravimetric set-up. Main image, microbalance with our miniature container being weighed (its position is indicated by a dashed square). Image to the right, the container is open and the Si nitride wafer (that is clamped between the O-rings during measurements) is removed. c, Examples of water evaporation through apertures of different diameters, D (colour coded) d, Dependence of the evaporation rate on D (error bars, s.d.). Red line, best linear fit. Inset, optical micrograph (natural colour) of an aperture of 30 μm diameter, which is etched in a Si nitride membrane.
Extended Data Figure 7 Molecular dynamics simulations of water flow through graphene slits.
a, Our capillaries are filled with water and the driving pressure is determined by evaporation of the extended meniscus that appears at the capillary mouth. The meniscus is sketched in the drawing, showing a thin film of water propagating along the graphite surface26. b, MD set-up with the simulation box indicated by the black lines. The particular snapshot is for N = 4. Dark grey balls represent carbon atoms arranged into graphene planes. Red and light grey spheres show oxygen and hydrogen atoms of water molecules. c, Simulated slip length δ and water flux Q as a function of N. In the case of NEMD, δ (blue symbols) was calculated from the simulated Q (blue bars) using equation (1). Using δ found from the EMD simulations (grey symbols) and the pressure gradient of 1015 Pa m−1, equation (1) yields Q shown by the grey bars.
Extended Data Figure 8 MD simulations of capillary pressure.
a, Our MD set-up for N = 4. Colour coding as in Extended Data Fig. 7b. Graphene planes to the right represent a graphite crystal with four atomic planes removed. The vertical graphene plane is used as a movable membrane to apply a compensating force to stop the water meniscus from propagating to the right. b, Main figure, simulated capillary pressures (symbols with s.d. error bars). The red curve shows the best fit for large N using P0 = 2σcos(ϕ)/h, which yields ϕ ≈ 80°. Blue and magenta curves show ΠvdW with the Hamaker constant A ≈ 115 zJ (ref. 53), and the entropic pressure due to changes in ρ, respectively. Dashed green curve, combined pressure from the three contributions. Inset, simulated density ρ of water confined between graphene sheets under external pressure of 1 bar.
Extended Data Figure 9 Micromechanical stability of graphene cavities.
Shown are snapshots of mono- and bilayer capillaries (left and right columns, respectively) after 100 ps of MD simulations. a–d, Capillaries with different thicknesses of graphite walls, respectively 2, 6, 20 and 40 graphene layers.
Rights and permissions
About this article
Cite this article
Radha, B., Esfandiar, A., Wang, F. et al. Molecular transport through capillaries made with atomic-scale precision. Nature 538, 222–225 (2016). https://doi.org/10.1038/nature19363
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature19363
This article is cited by
-
Fabrication of angstrom-scale two-dimensional channels for mass transport
Nature Protocols (2024)
-
Crystallization of molecular layers produced under confinement onto a surface
Nature Communications (2024)
-
Controllable van der Waals gaps by water adsorption
Nature Nanotechnology (2024)
-
Elastocapillarity-driven 2D nano-switches enable zeptoliter-scale liquid encapsulation
Nature Communications (2024)
-
Overcoming the permeability-selectivity challenge in water purification using two-dimensional cobalt-functionalized vermiculite membrane
Nature Communications (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.