Abstract
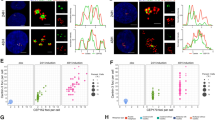
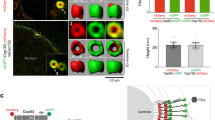
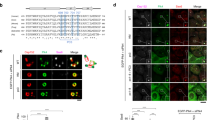
Controlling the number of its centrioles is vital for the cell, as supernumerary centrioles cause multipolar mitosis and genomic instability1,2. Normally, one daughter centriole forms on each mature (mother) centriole3,4; however, a mother centriole can produce multiple daughters within a single cell cycle5,6. The mechanisms that prevent centriole 'overduplication' are poorly understood. Here we use laser microsurgery to test the hypothesis that attachment of the daughter centriole to the wall of the mother inhibits formation of additional daughters7,8. We show that physical removal of the daughter induces reduplication of the mother in S-phase-arrested cells. Under conditions when multiple daughters form simultaneously on a single mother, all of these daughters must be removed to induce reduplication. The number of daughter centrioles that form during reduplication does not always match the number of ablated daughter centrioles. We also find that exaggeration of the pericentriolar material (PCM) by overexpression of the PCM protein pericentrin9 in S-phase-arrested CHO cells induces formation of numerous daughter centrioles. We propose that that the size of the PCM cloud associated with the mother centriole restricts the number of daughters that can form simultaneously.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Nigg, E. A. Centrosome aberrations: cause or consequence of cancer progression? Nature Rev. Cancer 2, 815–825 (2002).
Sluder, G. & Nordberg, J. J. The good, the bad and the ugly: the practical consequences of centrosome amplification. Curr. Opin. Cell Biol. 16, 49–54 (2004).
Tsou, M.-F. T. & Stearns, T. Controlling centrosome number: licenses and blocks. Curr. Opin. Cell Biol. 18, 74–78 (2006).
Duensing, A. et al. Centriole overduplication through the concurrent formation of multiple daughter centrioles at single maternal templates. Oncogene 26, 6280–6288 (2007).
Balczon, R. et al. Dissociation of centrosome replication events from cycles of DNA synthesis and mitotic division in hydroxyurea-arrested Chinese hamster ovary cells. J. Cell Biol. 130, 105–115 (1995).
Kleylein-Sohn, J. et al. Plk4-induced centriole biogenesis in human cells. Dev. Cell 13, 190–202 (2007).
Wong, C. & Stearns, T. Centrosome number is controlled by a centrosome-intrinsic block to reduplication. Nature Cell Biol. 5, 539–544 (2003).
Tsou, M. F. & Stearns, T. Mechanism limiting centrosome duplication to once per cell cycle. Nature 442, 947–951 (2006).
Dictenberg, J. B. et al. Pericentrin and γ-tubulin form a protein complex and are organized into a novel lattice at the centrosome. J. Cell Biol. 141, 163–174 (1998).
Bobinnec, Y. et al. Centriole disassembly in vivo and its effect on centrosome structure and function in vertebrate cells. J. Cell Biol. 143, 1575–1589 (1998).
Kuriyama, R. & Borisy, G. G. Centriole cycle in Chinese hamster ovary cells as determined by whole-mount electron microscopy. J. Cell Biol. 91, 814–821 (1981).
Vorobjev, I. A. & Chentsov, Y. S. Centrioles in the cell cycle. I. Epithelial cells. J. Cell Biol. 93, 938–949 (1982).
Paintrand, M., Moudjou, M., Delacroix, H. & Bornens, M. Centrosome organization and centriole architecture: their sensitivity to divalent cations. J. Struct. Biol. 108, 107–128 (1992).
Marshall, W. F. What is the function of centrioles? J. Cell. Biochem. 100, 916–922 (2007).
Vidwans, S. J., Wong, M. L. & O'Farrell, P. H. Anomalous centriole configurations are detected in Drosophila wing disc cells upon Cdk1 inactivation. J. Cell Sci. 116, 137–143 (2003).
Habedank, R., Stierhof, Y. D., Wilkinson, C. J. & Nigg, E. A. The Polo kinase Plk4 functions in centriole duplication. Nature Cell Biol. 7, 1140–1146 (2005).
Piel, M., Meyer, P., Khodjakov, A., Rieder, C. L. & Bornens, M. The respective contributions of the mother and daughter centrioles to centrosome activity and behavior in vertebrate cells. J. Cell Biol. 149, 317–330 (2000).
Khodjakov, A. et al. De novo formation of centrosomes in vertebrate cells arrested during S phase. J. Cell Biol. 158, 1171–1181 (2002).
La Terra, S. et al. The de novo centriole assembly pathway in HeLa cells: cell cycle progression and centriole assembly/maturation. J. Cell Biol. 168, 713–720 (2005).
Uetake, Y. et al. Cell cycle progression and de novo centriole assembly after centrosomal removal in untransformed human cells. J. Cell Biol. 176, 173–182 (2007).
Kim, H.-K. et al. De novo formation of basal bodies in Naegleria gruberi: regulation by phosphorylation. J. Cell Biol. 169, 719–724 (2005).
Dammermann, A. et al. Centriole assembly requires both centriolar and pericentriolar material proteins. Dev. Cell 7, 815–829 (2004).
Pelletier, L., O'Toole, E., Schwager, A., Hyman, A. A. & Muller-Reichert, T. Centriole assembly in Caenorhabditis elegans. Nature 444, 619–623 (2006).
Rodrigues-Martins, A., Riparbelli, M., Callaini, G., Glover, D. M. & Bettencourt-Dias, M. Revisiting the role of the mother centriole in centriole biogenesis. Science 316, 1046–1050 (2007).
Peel, N., Stevens, N. R., Basto, R. & Raff, J. W. Overexpressing centriole-replication proteins in vivo induces centriole overduplication and de novo formation. Curr. Biol. 17, 834–843 (2007).
Marshall, W. F., Vucica, Y. & Rosenbaum, J. L. Kinetics and regulations of de novo centriole assembly: implications for the mechanism of centriole duplication. Curr. Biol. 11, 308–317 (2001).
Young, A., Dictenberg, J. B., Purohit, A., Tuft, R. & Doxsey, S. J. Cytoplasmic dynein-mediated assembly of pericentrin and γ-tubulin onto centrosomes. Mol. Biol. Cell 11, 2047–2056 (2000).
Magidson, V., Loncarek, J., Hergert, P., Rieder, C. L. & Khodjakov, A. in Laser Manipulations of Cells and Tissues (eds. Berns, M. W. & Greulich, K. O.) 237–266 (Elsevier, 2007).
Purohit, A., Tynan, S. H., Vallee, R. B. & Doxsey, S. J. Direct interaction of pericentrin with cytoplasmic dynein light intermediate chain contributes to mitotic spindle organization. J. Cell Biol. 147, 481–492 (1999).
Strnad, P. et al. Regulated HsSAS-6 levels ensure formation of a single procentriole per centriole during the centrosome duplication cycle. Dev. Cell 13, 203–213 (2007).
Acknowledgements
We thank the members of our lab for fruitful discussions. Special thanks to Brian M. Davis and Igor B. Roninson for their help with construction of the centrin–GFP lentivirus and Yimin Dong for assistance with 3D reconstructions. We also thank Kip Sluder, Conly Rieder and Michael Koonce for critical comments on the manuscript. This work was supported by grants from the National Institutes of Health (GM GM59363) and the Human Frontiers Science Program (RGP0064). Construction of our laser microsurgery workstation was supported in part by a fellowship from Nikon/Marine Biological Laboratory (A. K.). We acknowledge use of the Wadsworth Center's Electron Microscopy Core Facility.
Author information
Authors and Affiliations
Contributions
Experiments were conducted by J. L.; P. H. was responsible for EM preparation and data collection; V. M. designed, assembled and maintained the laser microsurgery workstation; A. K. directed the work. Experiments were planned by J. L. and A. K.
Corresponding author
Supplementary information
Supplementary Information
Supplementary figures S1, S2, S3, S4, S5, S6, S7, S8, Movie legends and Supplementary text (PDF 1647 kb)
Supplementary Information
Supplementary Movie 1 (MOV 5029 kb)
Supplementary Information
Supplementary Movie 2 (MOV 1695 kb)
Supplementary Information
Supplementary Movie 3 (MOV 1135 kb)
Supplementary Information
Supplementary Movie 4 (MOV 11 kb)
Supplementary Information
Supplementary Movie 5 (MOV 17 kb)
Supplementary Information
Supplementary Movie 6 (MOV 41 kb)
Supplementary Information
Supplementary Movie 7 (MOV 78 kb)
Supplementary Information
Supplementary Movie 8 (MOV 231 kb)
Supplementary Information
Supplementary Movie 9 (MOV 4477 kb)
Supplementary Information
Supplementary Movie 10 (MOV 3736 kb)
Supplementary Information
Supplementary Movie 11 (MOV 5013 kb)
Rights and permissions
About this article
Cite this article
Loncarek, J., Hergert, P., Magidson, V. et al. Control of daughter centriole formation by the pericentriolar material. Nat Cell Biol 10, 322–328 (2008). https://doi.org/10.1038/ncb1694
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1694
This article is cited by
-
Mild replication stress causes premature centriole disengagement via a sub-critical Plk1 activity under the control of ATR-Chk1
Nature Communications (2023)
-
Centrosome amplification: a quantifiable cancer cell trait with prognostic value in solid malignancies
Cancer and Metastasis Reviews (2021)
-
Centrosome reduction in newly-generated tetraploid cancer cells obtained by separase depletion
Scientific Reports (2020)
-
Dynamics of centriole amplification in centrosome-depleted brain multiciliated progenitors
Scientific Reports (2019)
-
Selective autophagy maintains centrosome integrity and accurate mitosis by turnover of centriolar satellites
Nature Communications (2019)