Abstract
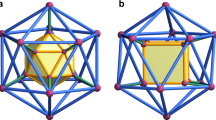
Platonic and Archimedean polyhedra, well-known to mathematicians, have been recently constructed by chemists at a molecular scale by defining the vertices and the edges with metal ions (M) and organic ligands (L), respectively. Here, we report the first synthesis of a concave-surface ‘stellated polyhedron’, constructed by extending the faces of its precursor polyhedron until they intersect, forming additional nodes. Our approach involves the formation of an M12L24 cuboctahedron core, the linkers of which each bear a pendant ligand site that is subsequently able to bind an additional metal centre to form the stellated M18L24 cuboctahedron. During this post-stellation process, the square faces of the M12L24 core are closed by coordination of the pendant moieties to the additional metal centres, but they are re-opened on removing these metals ions from the vertices. This behaviour is reminiscent of the analogous metal-triggered gate opening–closing switches found in spherical virus capsids.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Wenninger, M. J. Dual Models (Cambridge Univ. Press, 2003).
Fujita, M. et al. Self-assembly of ten molecules into nanometre-sized organic host frameworks. Nature 378, 469–471 (1995).
MacGillivray, L. R. & Atwood, J. L. A chiral spherical molecular assembly held together by 60 hydrogen bonds. Nature 389, 469–472 (1997).
Stang, P. J., Olenyuk, B., Muddiman, D. C. & Smith, R. D. Transition-metal-mediated rational design and self-assembly of chiral, nanoscale supramolecular polyhedra with unique T symmetry. Organometallics 16, 3094–3096 (1997).
Caulder, D. L., Powers, R. E., Parac, T. N. & Raymond, K. N. The self-assembly of a predesigned tetrahedral M4L6 supramolecular cluster. Angew. Chem. Int. Ed. 37, 1840–1843 (1998).
Takeda, N., Umemoto, K., Yamaguchi, K. & Fujita, M. A nanometre-sized hexahedral coordination capsule assembled from 24 components. Nature 398, 794–796 (1999).
Olenyuk, B., Whiteford, J. A., Fechtenkötter, A. & Stang, P. J. Self-assembly of nanoscale cuboctahedra by coordination chemistry. Nature 398, 796–799 (1999).
Olenyuk, B., Levin, M. D., Whiteford, J. A., Shield, J. E. & Stang, P. J. Self-assembly of nanoscopic dodecahedra from 50 predesigned components. J. Am. Chem. Soc. 121, 10434–10435 (1999).
Abrahams, B. F., Egan, S. J. & Robson, R. A very large metallosupramolecular capsule with cube-like 43 topology assembled from twelve Cu(II) centers and eight tri-bidentate tri-anionic ligands derived from 2,4,6-triphenylazo-1,3,5-trihydroxybenzene. J. Am. Chem. Soc. 121, 3535–3536 (1999).
Moulton, B., Lu, J., Mondal, A. & Zaworotko, M. J. Nanoballs: nanoscale faceted polyhedra with large windows and cavities. Chem. Commun. 863–864 (2001).
Eddaoudi, M. et al. Porous metal−organic polyhedra: 25 Å cuboctahedron constructed from 12 Cu2(CO2)4 paddle-wheel building blocks. J. Am. Chem. Soc. 123, 4368–4369 (2001).
Tominaga, M. et al. Finite, spherical coordination networks that self-organize from 36 small components. Angew. Chem. Int. Ed. 43, 5621–5625 (2004).
Sun, Q.-F. et al. Self-assembled M24L48 polyhedra and their sharp structural switch upon subtle ligand variation. Science 328, 1144–1147 (2010).
Leininger, S., Olenyuk, B. & Stang, P. J. Self-assembly of discrete cyclic nanostructures mediated by transition metals. Chem. Rev. 100, 853–908 (2000).
Fujita, M. et al. Molecular paneling via coordination. Chem. Commun. 509–518 (2001).
Caulder, D. L. & Raymond, K. N. Supermolecules by design. Acc. Chem. Res. 32, 975–982 (1999).
Müller, A., Kögerler, P. & Dress, A. W. M. Giant metal-oxide-based spheres and their topology: from pentagonal blocks to keplerates and unusual spin systems. Coord. Chem. Rev. 222, 193–218 (2001).
Cotton, F. A., Lin, C. & Murillo, C. A. Supramolecular arrays based on dimetal building units. Acc. Chem. Res. 34, 759–771 (2001).
Ronson, T. K., Fisher, J., Harding, L. P. & Hardie M. J. Star-burst prisms with cyclotriveratrylene-type ligands: a [Pd6L8]12+ stella octangular structure. Angew. Chem. Int. Ed. 46, 9086–9088 (2007).
Hong, M. et al. A nanometer-sized metallosupramolecular cube with O h symmetry. J. Am. Chem. Soc. 122, 4819–4820 (2000).
Duriska, M. B. et al. A nanoscale molecular switch triggered by thermal, light, and guest perturbation. Angew. Chem. Int. Ed. 48, 2549–2552 (2009).
Duriska, M. B. et al. Systematic metal variation and solvent and hydrogen-gas storage in supramolecular nanoballs. Angew. Chem. Int. Ed. 48, 8919–8922 (2009).
Chand, D. K., Biradha, K., Fujita, M., Sakamoto, S. & Yamaguchi, K. A molecular sphere of octahedral symmetry. Chem. Commun. 2486–2487 (2002).
Moon, D. et al. Face-driven corner-linked octahedral nanocages: M6L8 cages formed by C3-symmetric triangular facial ligands linked via C4-symmetric square tetratopic PdII ions at truncated octahedron corners. J. Am. Chem. Soc. 128, 3530–3531 (2006).
Hiraoka, S. et al. Isostructural coordination capsules for a series of 10 different d5–d10 transition-metal ions. Angew. Chem. Int. Ed. 45, 6488–6491 (2006).
Ronson, T. K. et al. Stellated polyhedral assembly of a topologically complicated Pd4L4 ‘Solomon cube’. Nature Chem. 1, 212–216 (2009).
Yamaguchi, K. Cold-spray ionization mass spectrometry: principle and applications. J. Mass Spectrom. 38, 473–490 (2003).
Sato, S., Ishido, Y. & Fujita, M. Remarkable stabilization of M12L24 spherical frameworks through the cooperation of 48 Pd(II)-pyridine interactions. J. Am. Chem. Soc. 131, 6064–6065 (2009).
Hsu, C. H., Sehgal, O. P. & Pickett, E. E. Stabilizing effect of divalent metal ions on virions of southern bean mosaic virus. Virology 69, 587–595 (1976).
Rayment, I., Johnson J. E. & Rossmann, M. G. Metal-free southern bean mosaic virus crystals. J. Biol. Chem. 254, 5243–5245 (1979).
Robinson, I. K. & Harrison, S. C. Structure of the expanded state of tomato bushy stunt virus. Nature 297, 563–568 (1982).
Speir, J. A., Munshi, S., Wang, G., Baker, T. S. & Johnson, J. E. Structures of the native and swollen forms of cowpea chlorotic mottle virus determined by X-ray crystallography and cryo-electron microscopy. Structure 3, 63–78 (1995).
Spek, A. L. & Van Der Sluis, P. BYPASS: an effective method for the refinement of crystal structures containing disordered solvent regions. Acta Crystallogr. A 46, 194–201 (1990).
Spek A. L. Structure validation in chemical crystallography. Acta Crystallogr. D 65, 148–155 (2009).
Acknowledgements
This research was supported by the CREST project of the Japan Science and Technology Agency (JST), for which M.F. is the principal investigator, and also in part by KAKENHI, MEXT. Synchrotron X-ray diffraction studies were performed at KEK and SPring-8. The authors thank T. Ozeki for valuable suggestions on the refinements of the X-ray structures.
Author information
Authors and Affiliations
Contributions
M.F. and Q.-F.S. conceived and designed the experiments and wrote the manuscript. Q.-F.S. performed the experiments and analysed the data. S.S. contributed the diffraction and NMR studies and participated in discussions throughout.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 2391 kb)
Supplementary information
Supplementary information (CIF 20 kb)
Supplementary information
Supplementary information (CIF 22 kb)
Rights and permissions
About this article
Cite this article
Sun, QF., Sato, S. & Fujita, M. An M18L24 stellated cuboctahedron through post-stellation of an M12L24 core. Nature Chem 4, 330–333 (2012). https://doi.org/10.1038/nchem.1285
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1285
This article is cited by
-
Post-synthetic modifications of metal–organic cages
Nature Reviews Chemistry (2022)
-
Crystallography and Mass-Spectrometry of Heptanuclear Disk Mn(II) Cluster
Journal of Cluster Science (2022)
-
4.8 nm Concave {M72} (M=Co, Ni, Fe) metal-organic polyhedra capped by 18 calixarenes
Science China Chemistry (2021)
-
Assembly of Dy60 and Dy30 cage-shaped nanoclusters
Communications Chemistry (2020)
-
Equi–size nesting of Platonic and Archimedean metal–organic polyhedra into a twin capsid
Nature Communications (2020)