Abstract
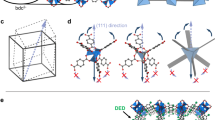
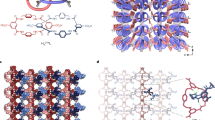
The mechanical flexibility of coordination frameworks can lead to a range of highly anomalous structural behaviours. Here, we demonstrate the extreme compressibility of the LnFe(CN)6 frameworks (Ln = Ho, Lu or Y), which reversibly compress by 20% in volume under the relatively low pressure of 1 GPa, one of the largest known pressure responses for any crystalline material. We delineate in detail the mechanism for this high compressibility, where the LnN6 units act like torsion springs synchronized by rigid Fe(CN)6 units performing the role of gears. The materials also show significant negative linear compressibility via a cam-like effect. The torsional mechanism is fundamentally distinct from the deformation mechanisms prevalent in other flexible solids and relies on competition between locally unstable metal coordination geometries and the constraints of the framework connectivity, a discovery that has implications for the strategic design of new materials with exceptional mechanical properties.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Anderson, M. S. & Swenson, C. A. Experimental compressions for sodium, potassium, and rubidium metals to 20 kbar from 4.2 to 300 K. Phys. Rev. B 28, 5395–5418 (1983).
Anderson, M. S. & Swenson, C. A. Experimental equations of state for cesium and lithium metals to 20 kbar and the high-pressure behavior of the alkali-metals. Phys. Rev. B 31, 668–680 (1985).
Packard, J. R. & Swenson, C. A. An experimental equation of state for solid xenon. J. Phys. Chem. Solids 24, 1405–1418 (1963).
Serre, C. et al. Role of solvent–host interactions that lead to very large swelling of hybrid frameworks. Science 315, 1828–1831 (2007).
Lee, Y., Vogt, T., Hriljac, J. A., Parise, J. B. & Artioli, G. Pressure-induced volume expansion of zeolites in the natrolite family. J. Am. Chem. Soc. 124, 5466–5475 (2002).
Goodwin, A. L., Keen, D. A. & Tucker, M. G. Large negative linear compressibility of Ag3[Co(CN)6]. Proc. Natl Acad. Sci. USA 105, 18708–18713 (2008).
Chapman, K. W., Halder, G. J. & Chupas, P. J. Pressure-induced amorphization and porosity modification in a metal–organic framework. J. Am. Chem. Soc. 131, 17546–17547 (2009).
Bennett, T. D. et al. Reversible pressure-induced amorphization of a zeolitic imidazolate framework (ZIF-4). Chem. Commun. 47, 7983–7985 (2011).
Cairns, A. B., Thompson, A. L., Tucker, M. G., Haines, J. & Goodwin, A. L. Rational design of materials with extreme negative compressibility: selective soft-mode frustration in KMn[Ag(CN)2]3 . J. Am. Chem. Soc. 134, 4454–4456 (2011).
Cairns, A. B. et al. Giant negative linear compressibility in zinc dicyanoaurate. Nature Mater. 12, 212–216 (2013).
Cai, W. & Katrusiak, A. Giant negative linear compression positively coupled to massive thermal expansion in a metal–organic framework. Nature Commun. 5, 4337 (2014).
Cairns, A. B. & Goodwin, A. Negative linear compressibility. Phys. Chem. Chem. Phys. 17, 20449–20465 (2015).
Goodwin, A. L. & Kepert, C. J. Negative thermal expansion and low-frequency modes in cyanide-bridged framework materials. Phys. Rev. B 71, 140301 (2005).
Chapman, K. W. & Chupas, P. J. Pressure enhancement of negative thermal expansion behavior and induced framework softening in zinc cyanide. J. Am. Chem. Soc. 129, 10090–10091 (2007).
Phillips, A. E., Goodwin, A. L., Halder, G. J., Southon, P. D. & Kepert, C. J. Nanoporosity and exceptional negative thermal expansion in single-network cadmium cyanide. Angew. Chem. Int. Ed. 47, 1396–1399 (2008).
Liu, Y. et al. Reversible structural transition in MIL-53 with large temperature hysteresis. J. Am. Chem. Soc. 130, 11813–11818 (2008).
Das, D., Jacobs, T. & Barbour, L. J. Exceptionally large positive and negative anisotropic thermal expansion of an organic crystalline material. Nature Mater. 9, 36–39 (2010).
Duyker, S. G., Peterson, V. K., Kearley, G. J., Ramirez-Cuesta, A. J. & Kepert, C. J. Negative thermal expansion in LnCo(CN)6 (Ln=La, Pr, Sm, Ho, Lu, Y): mechanisms and compositional trends. Angew. Chem. Int. Ed. 52, 5266–5270 (2013).
Yao, Z.-S. et al. Molecular motor-driven abrupt anisotropic shape change in a single crystal of a Ni complex. Nature Chem. 6, 1079–1083 (2014).
Fortes, A. D., Suard, E. & Knight, K. S. Negative linear compressibility and massive anisotropic thermal expansion in methanol monohydrate. Science 331, 742–746 (2011).
Shepherd, H. J. et al. Antagonism between extreme negative linear compression and spin crossover in [Fe(dpp)2(NCS)2]⋅py. Angew. Chem. Int. Ed. 51, 3910–3914 (2012).
Cai, W., He, J., Li, W. & Katrusiak, A. Anomalous compression of a weakly CHO bonded nonlinear optical molecular crystal. J. Mater. Chem. C 2, 6471–6476 (2014).
Pretsch, T., Chapman, K. W., Halder, G. J. & Kepert, C. J. Dehydration of the nanoporous coordination framework ErIII[CoIII(CN)6]·4(H2O): single crystal to single crystal transformation and negative thermal expansion in ErIII[CoIII(CN)6]. Chem. Commun. 1857–1859 (2006).
Kepert, D. L. Inorganic Stereochemistry (Springer-Verlag, 1982).
Kono, R. The dynamic bulk viscosity of polystyrene and polymethyl methacrylate. J. Phys. Soc. Jpn 15, 718–725 (1960).
Neimark, A. V. et al. Structural transitions in MIL-53 (Cr): view from outside and inside. Langmuir 27, 4734–4741 (2011).
Duyker, S. G. et al. Topotactic structural conversion and hydration-dependent thermal expansion in robust LnMIII(CN)6·nH2O and flexible ALnFeII(CN)6·nH2O frameworks (A = Li, Na, K; Ln = La-Lu, Y; M = Co, Fe; 0 ≤ n ≤ 5). Chem. Sci. 5, 3409–3417 (2014).
Serra-Crespo, P. et al. Experimental evidence of negative linear compressibility in the MIL-53 metal–organic framework family. CrystEngComm 17, 276–280 (2015).
Baughman, R. H., Stafström, S., Cui, C. & Dantas, S. O. Materials with negative compressibilities in one or more dimensions. Science 279, 1522–1524 (1998).
Kunkely, H. & Vogler, A. Optical properties of GdIII[MIII(CN)6] with M=Cr and Co. Phosphorescence from ligand-field states of [M(CN)6]3− under ambient conditions. Inorg. Chem. Commun. 7, 770–772 (2004).
Studer, A. J., Hagen, M. E. & Noakes, T. J. Wombat: the high-intensity powder diffractometer at the OPAL reactor. Physica B 385–386, 1013–1015 (2006).
Brown, J. M. The NaCl pressure standard. J. Appl. Phys. 86, 5801 (1999).
Besson, J. M. et al. Neutron powder diffraction above 10 GPa. Physica B 180, 907–910 (1992).
Larson, A. C. & Von Dreele, R. B. General Structure Analysis System (GSAS), Los Alamos National Laboratory Report LAUR 86-748 (Los Alamos National Laboratory, 2000).
Toby, B. H. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 34, 210–213 (2001).
Cliffe, M. J. & Goodwin, A. L. PASCal: a principal axis strain calculator for thermal expansion and compressibility determination. J. Appl. Crystallogr. 45, 1321–1329 (2012).
Kresse, G. & Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Minisini, B., Hadj, L. E., Fomena, M. L., Garderen, N. V. & Tsobnang, F. A density functional study of the pressure induced phase transition in LiYF4 . J. Phys. Condens. Matter 18, 2429 (2006).
Bouzid, A. et al. in Molecular Dynamics Simulations of Disordered Materials (eds Massobrio, C., Du, J., Bernasconi, M. & Salmon, P. S. ) Ch. 12, 313–344 (Springer Series in Materials Science 215, Springer International, 2015).
Kearley, G. J., Johnson, M. R. & Tomkinson, J. Intermolecular interactions in solid benzene. J. Chem. Phys. 124, 044514 (2006).
Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 27, 1787–1799 (2006).
Delley, B. An all-electron numerical method for solving the local density functional for polyatomic molecules. J. Chem. Phys. 92, 508–517 (1990).
Delley, B. From molecules to solids with the DMol3 approach. J. Chem. Phys. 113, 7756–7764 (2000).
Acknowledgements
C.J.K. acknowledges financial support from the Australian Research Council. The neutron scattering experiments were performed under OPAL proposal P2455. The authors thank the Bragg Institute sample environment team for their assistance.
Author information
Authors and Affiliations
Contributions
S.G.D., V.K.P. and C.J.K. conceived the study, analysed and interpreted the data, and wrote the paper. S.G.D., V.K.P., G.J.K. and A.J.S. performed the experiments and DFT calculations.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 2044 kb)
Supplementary movie
Supplementary movie 1 (AVI 4483 kb)
Rights and permissions
About this article
Cite this article
Duyker, S., Peterson, V., Kearley, G. et al. Extreme compressibility in LnFe(CN)6 coordination framework materials via molecular gears and torsion springs. Nature Chem 8, 270–275 (2016). https://doi.org/10.1038/nchem.2431
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2431
This article is cited by
-
Exploration of glassy state in Prussian blue analogues
Nature Communications (2022)
-
Atomic resolution of structural changes in elastic crystals of copper(II) acetylacetonate
Nature Chemistry (2018)
-
Continuous negative-to-positive tuning of thermal expansion achieved by controlled gas sorption in porous coordination frameworks
Nature Communications (2018)