Abstract
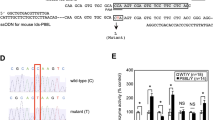
Mucopolysaccharidosis type I (MPS I), caused by mutations in the gene encoding α-L-iduronidase (IDUA), is one of approximately 70 genetic disorders collectively known as the lysosomal storage diseases. To gain insight into the basis for MPS I, we crystallized human IDUA produced in an Arabidopsis thaliana cgl mutant. IDUA consists of a TIM barrel domain containing the catalytic site, a β-sandwich domain and a fibronectin-like domain. Structures of IDUA bound to iduronate analogs illustrate the Michaelis complex and reveal a 2,5B conformation in the glycosyl-enzyme intermediate, which suggest a retaining double displacement reaction involving the nucleophilic Glu299 and the general acid/base Glu182. Unexpectedly, the N-glycan attached to Asn372 interacts with iduronate analogs in the active site and is required for enzymatic activity. Finally, these IDUA structures and biochemical analysis of the disease-relevant P533R mutation have enabled us to correlate the effects of mutations in IDUA to clinical phenotypes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
20 September 2013
In the version of this article initially published online, in the Online Methods section 'Synthesized IDUA inhibitors', two different chemical names were provided for 5F-IdoAF, and a complete name for 2F-IdoAF was inadvertently omitted. Additionally, in the legend for Figure 5, one instance of mg−1 was mistakenly written as mg−. The page numbers for reference 6 were also incorrect. These errors have been corrected for the print, PDF and HTML versions of this article.
References
Clarke, L.A. The mucopolysaccharidoses: a success of molecular medicine. Expert Rev. Mol. Med. 10, e1 (2008).
Roubicek, M., Gehler, J. & Spranger, J. The clinical spectrum of α-L-iduronidase deficiency. Am. J. Med. Genet. 20, 471–481 (1985).
Valenzano, K.J. et al. Identification and characterization of pharmacological chaperones to correct enzyme deficiencies in lysosomal storage disorders. Assay Drug Dev. Technol. 9, 213–235 (2011).
Tropak, M.B. et al. Identification of pharmacological chaperones for Gaucher disease and characterization of their effects on β-glucocerebrosidase by hydrogen/deuterium exchange mass spectrometry. ChemBioChem 9, 2650–2662 (2008).
Yu, Z., Sawkar, A.R. & Kelly, J.W. Pharmacologic chaperoning as a strategy to treat Gaucher disease. FEBS J. 274, 4944–4950 (2007).
Neufeld, E.F. & Muenzer, J. The Metabolic and Molecular Bases of Inherited Disease Vol. III (eds. Scriver, C.R. et al.) 3421–3452 (McGraw-Hill, New York, 2001).
Terlato, N.J. & Cox, G.F. Can mucopolysaccharidosis type I disease severity be predicted based on a patient's genotype? A comprehensive review of the literature. Genet. Med. 5, 286–294 (2003).
Nieman, C.E. et al. Family 39 α-L-iduronidases and β-D-xylosidases react through similar glycosyl-enzyme intermediates: identification of the human iduronidase nucleophile. Biochemistry 42, 8054–8065 (2003).
Yang, J.K. et al. Crystal structure of β-D-xylosidase from Thermoanaerobacterium saccharolyticum, a family 39 glycoside hydrolase. J. Mol. Biol. 335, 155–165 (2004).
Altschul, S.F., Gish, W., Miller, W., Myers, E.W. & Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 215, 403–410 (1990).
Rempel, B.P., Clarke, L.A. & Withers, S.G. A homology model for human α-L-iduronidase: insights into human disease. Mol. Genet. Metab. 85, 28–37 (2005).
Zhao, K.W., Faull, K.F., Kakkis, E.D. & Neufeld, E.F. Carbohydrate structures of recombinant human α-L-iduronidase secreted by Chinese hamster ovary cells. J. Biol. Chem. 272, 22758–22765 (1997).
He, X. et al. Characterization and downstream mannose phosphorylation of human recombinant α-L-iduronidase produced in Arabidopsis complex glycan-deficient (cgl) seeds. Plant Biotechnol J. 10.1111/pbi.12096 (31 July 2013).
Vocadlo, D.J., MacKenzie, L.F., He, S., Zeikus, G.J. & Withers, S.G. Identification of glu-277 as the catalytic nucleophile of Thermoanaerobacterium saccharolyticum β-xylosidase using electrospray MS. Biochem. J. 335, 449–455 (1998).
Armand, S. et al. Stereochemical course and reaction products of the action of β-xylosidase from Thermoanaerobacterium saccharolyticum strain B6A-RI. Eur. J. Biochem. 236, 706–713 (1996).
Rostkowski, M., Olsson, M.H., Sondergaard, C.R. & Jensen, J.H. Graphical analysis of pH-dependent properties of proteins predicted using PROPKA. BMC Struct. Biol. 11, 6 (2011).
Maita, N. et al. Human α-L-iduronidase uses its own N-glycan as a substrate-binding and catalytic module. Proc. Natl. Acad. Sci. USA 110, 14628–14633 (2013).
Hopwood, J.J. & Muller, V. Diagnostic enzymology of α-L-iduronidase with special reference to a sulfated disaccharide derived from heparin. Clin. Sci. (Lond.) 62, 193–201 (1982).
Yogalingam, G. et al. Identification and molecular characterization of α-L-iduronidase mutations present in mucopolysaccharidosis type I patients undergoing enzyme replacement therapy. Hum. Mutat. 24, 199–207 (2004).
Clements, P.R., Muller, V. & Hopwood, J.J. Human α-L-iduronidase. 2. Catalytic properties. Eur. J. Biochem. 152, 29–34 (1985).
Vocadlo, D.J., Davies, G.J., Laine, R. & Withers, S.G. Catalysis by hen egg-white lysozyme proceeds via a covalent intermediate. Nature 412, 835–838 (2001).
Davies, G.J., Planas, A. & Rovira, C. Conformational analyses of the reaction coordinate of glycosidases. Acc. Chem. Res. 45, 308–316 (2012).
Koshland, D.E. Stereochemistry and the mechanism of enzymatic reactions. Biol. Rev. Camb. Philos. Soc. 28, 416–436 (1953).
Heightman, T.D. & Vasella, A.T. Recent insights into inhibition, structure, and mechanism of configuration-retaining glycosidases. Angew. Chem. Int. Edn Engl. 38, 750–770 (1999).
Sabini, E. et al. Catalysis and specificity in enzymatic glycoside hydrolysis: a 2,5B conformation for the glycosyl-enzyme intermediate revealed by the structure of the Bacillus agaradhaerens family 11 xylanase. Chem. Biol. 6, 483–492 (1999).
Sidhu, G. et al. Sugar ring distortion in the glycosyl-enzyme intermediate of a family G/11 xylanase. Biochemistry 38, 5346–5354 (1999).
Matte, U. et al. Identification and characterization of 13 new mutations in mucopolysaccharidosis type I patients. Mol. Genet. Metab. 78, 37–43 (2003).
Alif, N. et al. Mucopolysaccharidosis type I: characterization of a common mutation that causes Hurler syndrome in Moroccan subjects. Ann. Hum. Genet. 63, 9–16 (1999).
Laradi, S. et al. Mucopolysaccharidosis I: α-L-iduronidase mutations in three Tunisian families. J. Inherit. Metab. Dis. 28, 1019–1026 (2005).
Scott, H.S. et al. α-L-Iduronidase mutations (Q70X and P533R) associate with a severe Hurler phenotype. Hum. Mutat. 1, 333–339 (1992).
Bunge, S. et al. Genotype-phenotype correlations in mucopolysaccharidosis type I using enzyme kinetics, immunoquantification and in vitro turnover studies. Biochim. Biophys. Acta 1407, 249–256 (1998).
Bashyal, B.P., Chow, H.-F., Fellows, L.E. & Fleet, G.W.J. The synthesis of polyhydroxylated amino acids from glucuronolactone: enantiospecific synthesis of 2S,3R,4R,5S-trihydroxypipecolic acid, 2R,3R,4R,5S-trihydroxypipecolic acid and 2R,3R,4R-dihydroxyproline. Tetrahedron 43, 415–422 (1987).
Downing, W.L. et al. Synthesis of enzymatically active human α-L-iduronidase in Arabidopsis cgl (complex glycan-deficient) seeds. Plant Biotechnol. J. 4, 169–181 (2006).
He, X. et al. Production of α-L-iduronidase in maize for the potential treatment of a human lysosomal storage disease. Nat. Commun. 3, 1062 (2012).
He, X. et al. Influence of an ER-retention signal on the N-glycosylation of recombinant human α-L-iduronidase generated in seeds of Arabidopsis. Plant Mol. Biol. 79, 157–169 (2012).
Ruth, L., Eisenberg, D. & Neufeld, E.F. α-L-iduronidase forms semi-crystalline spherulites with amyloid-like properties. Acta Crystallogr. D Biol. Crystallogr. 56, 524–528 (2000).
Kabsch, W. Xds. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 (2010).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
Adams, P.D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Langer, G., Cohen, S.X., Lamzin, V.S. & Perrakis, A. Automated macromolecular model building for X-ray crystallography using ARP/wARP version 7. Nat. Protoc. 3, 1171–1179 (2008).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Winn, M.D. et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. D Biol. Crystallogr. 67, 235–242 (2011).
Murshudov, G.N. et al. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr. D Biol. Crystallogr. 67, 355–367 (2011).
Word, J.M., Lovell, S.C., Richardson, J.S. & Richardson, D.C. Asparagine and glutamine: using hydrogen atom contacts in the choice of side-chain amide orientation. J. Mol. Biol. 285, 1735–1747 (1999).
McCoy, A.J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Winn, M.D., Murshudov, G.N. & Papiz, M.Z. Macromolecular TLS refinement in REFMAC at moderate resolutions. Methods Enzymol. 374, 300–321 (2003).
Painter, J. & Merritt, E.A. Optimal description of a protein structure in terms of multiple groups undergoing TLS motion. Acta Crystallogr. D Biol. Crystallogr. 62, 439–450 (2006).
Laskowski, R.A., Macarthur, M.W., Moss, D.S. & Thornton, J.M. PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 26, 283–291 (1993).
Chakrabarty, R. et al. PSITE vectors for stable integration or transient expression of autofluorescent protein fusions in plants: probing Nicotiana benthamiana–virus interactions. Mol. Plant Microbe Interact. 20, 740–750 (2007).
Babajani, G., Tropak, M.B., Mahuran, D.J. & Kermode, A.R. Pharmacological chaperones facilitate the post-ER transport of recombinant N370S mutant β-glucocerebrosidase in plant cells: evidence that N370S is a folding mutant. Mol. Genet. Metab. 106, 323–329 (2012).
Acknowledgements
This paper is dedicated to the memory of John Colter (1923–2013), chair of the Department of Biochemistry, University of Alberta, from 1961 to 1987. We thank S. Khan for his technical assistance during in-house data collection and the staff at the Canadian Light Source in Saskatoon and the Stanford Synchrotron Radiation Lightsource for their assistance in the data collection. We are grateful for the initial work done by K. Bateman on the growth of the monoclinic form of IDUA crystals, for synthetic work performed by A. Wong and for the expression and purification of CBM-PNGase-F by E. Kwan. We also thank J. Hopwood for the monoclonal antibody to human IDUA. M.N.G.J. and A.R.K. are grateful for the funding support from the Canadian Institutes for Health Research (grant no. MOP123222). H.B. thanks Alberta Innovates Health Solution for the fellowship support. E.D.G.-B. thanks the Canadian Institute of Health Research for a postdoctoral fellowship. A.R.K. is grateful for funding support from the Canadian Society for Mucopolysaccharide and Related Diseases and to the Michael Smith Foundation for Health Research for the Senior Scholar Award (award no. CI-SSH-01915(07-1)).
Author information
Authors and Affiliations
Contributions
X.H. and A.R.K. performed protein production and purification and the comparative kinetics work related to IDUA deglycosylation and IDUAP533R. E.D.G.-B. performed chemical synthesis and other enzyme kinetics. H.B. crystallized the protein. H.B. and J.Y. collected the diffraction data and carried out structure determination. H.B., J.Y. and M.N.G.J. analyzed the data and wrote the paper with contributions from X.H., A.R.K., S.G.W. and E.D.G.-B. M.N.G.J. and A.R.K. supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Note, Supplementary Figures 1–3 and Supplementary Tables 1–4. (PDF 479 kb)
Rights and permissions
About this article
Cite this article
Bie, H., Yin, J., He, X. et al. Insights into mucopolysaccharidosis I from the structure and action of α-L-iduronidase. Nat Chem Biol 9, 739–745 (2013). https://doi.org/10.1038/nchembio.1357
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1357
This article is cited by
-
c.1898C>G/p.Ser633Trp Mutation in Alpha-l-Iduronidase: Clinical and Structural Implications
The Protein Journal (2021)
-
A novel compound mutation in alpha-L-iduronidase gene causes mucopolysaccharidosis type I
Journal of Genetics (2019)
-
Genetic testing of Mucopolysaccharidoses disease using multiplex PCR- based panels of STR markers: in silico analysis of novel mutations
Metabolic Brain Disease (2019)
-
Phenotype prediction for mucopolysaccharidosis type I by in silico analysis
Orphanet Journal of Rare Diseases (2017)
-
N-glycan structures and downstream mannose-phosphorylation of plant recombinant human alpha-l-iduronidase: toward development of enzyme replacement therapy for mucopolysaccharidosis I
Plant Molecular Biology (2017)