Abstract
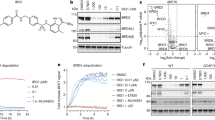
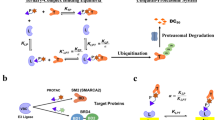
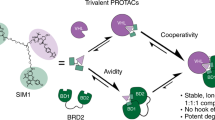
Inducing macromolecular interactions with small molecules to activate cellular signaling is a challenging goal. PROTACs (proteolysis-targeting chimeras) are bifunctional molecules that recruit a target protein in proximity to an E3 ubiquitin ligase to trigger protein degradation. Structural elucidation of the key ternary ligase–PROTAC–target species and its impact on target degradation selectivity remain elusive. We solved the crystal structure of Brd4 degrader MZ1 in complex with human VHL and the Brd4 bromodomain (Brd4BD2). The ligand folds into itself to allow formation of specific intermolecular interactions in the ternary complex. Isothermal titration calorimetry studies, supported by surface mutagenesis and proximity assays, are consistent with pronounced cooperative formation of ternary complexes with Brd4BD2. Structure-based-designed compound AT1 exhibits highly selective depletion of Brd4 in cells. Our results elucidate how PROTAC-induced de novo contacts dictate preferential recruitment of a target protein into a stable and cooperative complex with an E3 ligase for selective degradation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Huang, X. & Dixit, V.M. Drugging the undruggables: exploring the ubiquitin system for drug development. Cell Res. 26, 484–498 (2016).
Petzold, G., Fischer, E.S. & Thomä, N.H. Structural basis of lenalidomide-induced CK1α degradation by the CRL4(CRBN) ubiquitin ligase. Nature 532, 127–130 (2016).
Matyskiela, M.E. et al. A novel cereblon modulator recruits GSPT1 to the CRL4(CRBN) ubiquitin ligase. Nature 535, 252–257 (2016).
Lai, A.C. & Crews, C.M. Induced protein degradation: an emerging drug discovery paradigm. Nat. Rev. Drug Discov. 16, 101–114 (2016).
Sakamoto, K.M. et al. Protacs: chimeric molecules that target proteins to the Skp1-Cullin-F box complex for ubiquitination and degradation. Proc. Natl. Acad. Sci. USA 98, 8554–8559 (2001).
Winter, G.E. et al. Drug development. Phthalimide conjugation as a strategy for in vivo target protein degradation. Science 348, 1376–1381 (2015).
Zengerle, M., Chan, K.-H. & Ciulli, A. Selective small molecule induced degradation of the BET bromodomain protein BRD4. ACS Chem. Biol. 10, 1770–1777 (2015).
Lu, J. et al. Hijacking the E3 ubiquitin ligase cereblon to efficiently target BRD4. Chem. Biol. 22, 755–763 (2015).
Bondeson, D.P. et al. Catalytic in vivo protein knockdown by small-molecule PROTACs. Nat. Chem. Biol. 11, 611–617 (2015).
Deshaies, R.J. Protein degradation: prime time for PROTACs. Nat. Chem. Biol. 11, 634–635 (2015).
Toure, M. & Crews, C.M. Small-molecule PROTACS: new approaches to protein degradation. Angew. Chem. Int. Edn Engl. 55, 1966–1973 (2016).
Lai, A.C. et al. Modular PROTAC design for the degradation of oncogenic BCR-ABL. Angew. Chem. Int. Edn Engl. 55, 807–810 (2016).
Filippakopoulos, P. et al. Selective inhibition of BET bromodomains. Nature 468, 1067–1073 (2010).
Galdeano, C. et al. Structure-guided design and optimization of small molecules targeting the protein-protein interaction between the von Hippel-Lindau (VHL) E3 ubiquitin ligase and the hypoxia inducible factor (HIF) alpha subunit with in vitro nanomolar affinities. J. Med. Chem. 57, 8657–8663 (2014).
Frost, J. et al. Potent and selective chemical probe of hypoxic signalling downstream of HIF-α hydroxylation via VHL inhibition. Nat. Commun. 7, 13312 (2016).
Zuber, J. et al. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature 478, 524–528 (2011).
Nicodeme, E. et al. Suppression of inflammation by a synthetic histone mimic. Nature 468, 1119–1123 (2010).
Hon, W.-C. et al. Structural basis for the recognition of hydroxyproline in HIF-1 alpha by pVHL. Nature 417, 975–978 (2002).
Min, J.-H. et al. Structure of an HIF-1alpha -pVHL complex: hydroxyproline recognition in signaling. Science 296, 1886–1889 (2002).
Filippakopoulos, P. et al. Histone recognition and large-scale structural analysis of the human bromodomain family. Cell 149, 214–231 (2012).
Tanaka, M. et al. Design and characterization of bivalent BET inhibitors. Nat. Chem. Biol. 12, 1089–1096 (2016).
Whitty, A. Cooperativity and biological complexity. Nat. Chem. Biol. 4, 435–439 (2008).
Douglass, E.F. Jr., Miller, C.J., Sparer, G., Shapiro, H. & Spiegel, D.A. A comprehensive mathematical model for three-body binding equilibria. J. Am. Chem. Soc. 135, 6092–6099 (2013).
Eglen, R.M. et al. The use of AlphaScreen technology in HTS: current status. Curr. Chem. Genomics 1, 2–10 (2008).
Roberts, J.M. & Bradner, J.E. A bead-based proximity assay for BRD4 ligand discovery. Curr. Protoc. Chem. Biol. 7, 263–278 (2015).
Stebbins, C.E., Kaelin, W.G. Jr. & Pavletich, N.P. Structure of the VHL-ElonginC–ElonginB complex: implications for VHL tumor suppressor function. Science 284, 455–461 (1999).
Duda, D.M. et al. Structural insights into NEDD8 activation of cullin-RING ligases: conformational control of conjugation. Cell 134, 995–1006 (2008).
Zhou, M., Li, Q. & Wang, R. Current experimental methods for characterizing protein–protein interactions. ChemMedChem 11, 738–756 (2016).
Raina, K. et al. PROTAC-induced BET protein degradation as a therapy for castration-resistant prostate cancer. Proc. Natl. Acad. Sci. USA 113, 7124–7129 (2016).
Bulatov, E. & Ciulli, A. Targeting Cullin-RING E3 ubiquitin ligases for drug discovery: structure, assembly and small-molecule modulation. Biochem. J. 467, 365–386 (2015).
Fischer, E.S. et al. Structure of the DDB1-CRBN E3 ubiquitin ligase in complex with thalidomide. Nature 512, 49–53 (2014).
Fischer, E.S., Park, E., Eck, M.J. & Thomä, N.H. SPLINTS: small-molecule protein ligand interface stabilizers. Curr. Opin. Struct. Biol. 37, 115–122 (2016).
Tan, X. et al. Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature 446, 640–645 (2007).
Sheard, L.B. et al. Jasmonate perception by inositol-phosphate-potentiated COI1-JAZ co-receptor. Nature 468, 400–405 (2010).
Ito, T. et al. Identification of a primary target of thalidomide teratogenicity. Science 327, 1345–1350 (2010).
Lu, G. et al. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science 343, 305–309 (2014).
Krönke, J. et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science 343, 301–305 (2014).
Chamberlain, P.P. et al. Structure of the human Cereblon-DDB1-lenalidomide complex reveals basis for responsiveness to thalidomide analogs. Nat. Struct. Mol. Biol. 21, 803–809 (2014).
Pommier, Y. & Marchand, C. Interfacial inhibitors: targeting macromolecular complexes. Nat. Rev. Drug Discov. 11, 25–36 (2011).
Thiel, P., Kaiser, M. & Ottmann, C. Small-molecule stabilization of protein–protein interactions: an underestimated concept in drug discovery? Angew. Chem. Int. Edn Engl. 51, 2012–2018 (2012).
Illendula, A. et al. Chemical biology. A small-molecule inhibitor of the aberrant transcription factor CBFβ-SMMHC delays leukemia in mice. Science 347, 779–784 (2015).
Waring, M.J. et al. Potent and selective bivalent inhibitors of BET bromodomains. Nat. Chem. Biol. 12, 1097–1104 (2016).
Kabsch, W. XDS. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 (2010).
Evans, P.R. & Murshudov, G.N. How good are my data and what is the resolution? Acta Crystallogr. D Biol. Crystallogr. 69, 1204–1214 (2013).
Winn, M.D. et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. D Biol. Crystallogr. 67, 235–242 (2011).
McCoy, A.J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Murshudov, G.N. et al. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr. D Biol. Crystallogr. 67, 355–367 (2011).
Emsley, P., Lohkamp, B., Scott, W.G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Schüttelkopf, A.W. & van Aalten, D.M.F. PRODRG: a tool for high-throughput crystallography of protein-ligand complexes. Acta Crystallogr. D Biol. Crystallogr. 60, 1355–1363 (2004).
Chen, V.B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 (2010).
Krissinel, E. & Henrick, K. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 372, 774–797 (2007).
Laskowski, R.A. & Swindells, M.B. LigPlot+: multiple ligand-protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 51, 2778–2786 (2011).
Phillips, J.C. et al. Scalable molecular dynamics with NAMD. J. Comput. Chem. 26, 1781–1802 (2005).
Brooks, B.R. et al. CHARMM: the biomolecular simulation program. J. Comput. Chem. 30, 1545–1614 (2009).
Vanommeslaeghe, K. & MacKerell, A.D. Jr. Automation of the CHARMM general force field (CGenFF) I: bond perception and atom typing. J. Chem. Inf. Model. 52, 3144–3154 (2012).
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J.Mol.Graph. 14, 33–38 (1996).
Glykos, N.M. Software news and updates. Carma: a molecular dynamics analysis program. J. Comput. Chem. 27, 1765–1768 (2006).
Manza, L.L., Stamer, S.L., Ham, A.-J.L., Codreanu, S.G. & Liebler, D.C. Sample preparation and digestion for proteomic analyses using spin filters. Proteomics 5, 1742–1745 (2005).
Thompson, A. et al. Tandem mass tags: a novel quantification strategy for comparative analysis of complex protein mixtures by MS/MS. Anal. Chem. 75, 1895–1904 (2003).
Gilar, M., Olivova, P., Daly, A.E. & Gebler, J.C. Orthogonality of separation in two-dimensional liquid chromatography. Anal. Chem. 77, 6426–6434 (2005).
Acknowledgements
This work was supported by the European Research Council (ERC-2012-StG-311460 DrugE3CRLs Starting Grant to A.C.); the UK Biotechnology and Biological Sciences Research Council (BBSRC grant BB/J001201/2 to A.C.); the European Commission (H2020-MSCA-IF-2014-655516 Marie Skłodowska-Curie Actions Individual Fellowship to K.-H.C. and H2020-MSCA-IF-2015-806323 Marie Skłodowska-Curie Actions Individual Fellowship to X.L.); and the Wellcome Trust (Strategic Awards 100476/Z/12/Z for biophysics and drug discovery and 094090/Z/10/Z for structural biology and X-ray crystallography to the Division of Biological Chemistry and Drug Discovery). We are thankful to P. Fyfe for support with the in-house X-ray facility; L. Finn for support with tissue culture facility (MRC-PPU); the Ferguson lab for access to LI-COR equipment; T. Cardote (Ciulli lab, BCDD, SLS, Dundee) for the gift of full-length Cul2-Rbx1 and A. Knebel (MRC-PPU/DSTT) for the gift of E1 and E2 enzymes; the Division of Computational Biology for support with computational cluster; and to Diamond Light Source for beamtime (BAG proposal MX10071) and beamline support at beamline I04-1.
Author information
Authors and Affiliations
Contributions
A.C. conceived the idea and directed the project. M.S.G., X.L., A.T., K.-H.C. and A.C. designed the experiments and interpreted results. M.S.G., X.L., A.T., and K.-H.C. performed experiments. A.T. and M.Z. contributed to compound design and synthesized compounds. W.C. performed MS proteomics experiments under the supervision of D.J.L. M.S.G., X.L. and A.C. wrote the manuscript with input from all other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Tables 1–3 and Supplementary Figures 1–12 (PDF 4451 kb)
Supplementary Note
Chemistry (PDF 2033 kb)
Supplementary Data Set
Proteomic analysis of relative protein abundance in HeLa cells. Results are graphically represented in Figure 4f,g (XLSX 7377 kb)
Rights and permissions
About this article
Cite this article
Gadd, M., Testa, A., Lucas, X. et al. Structural basis of PROTAC cooperative recognition for selective protein degradation. Nat Chem Biol 13, 514–521 (2017). https://doi.org/10.1038/nchembio.2329
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.2329
This article is cited by
-
MZ1, a BRD4 inhibitor, exerted its anti-cancer effects by suppressing SDC1 in glioblastoma
BMC Cancer (2024)
-
DNA-encoded library-enabled discovery of proximity-inducing small molecules
Nature Chemical Biology (2024)
-
Development and crystal structures of a potent second-generation dual degrader of BCL-2 and BCL-xL
Nature Communications (2024)
-
CAND1 inhibits Cullin-2-RING ubiquitin ligases for enhanced substrate specificity
Nature Structural & Molecular Biology (2024)
-
Mechanism of millisecond Lys48-linked poly-ubiquitin chain formation by cullin-RING ligases
Nature Structural & Molecular Biology (2024)