Abstract
Targeting the human epidermal growth factor receptor type 2 (HER2) in breast cancer patients whose tumors overexpress HER2 has been clearly demonstrated to be effective in clinical trials with the monoclonal antibody trastuzumab. Not all patients, however, respond to trastuzumab therapy. Lapatinib is an oral receptor tyrosine kinase inhibitor that targets HER2 and the EGFR. Preclinical data reveal that lapatinib has activity in trastuzumab-resistant cell lines as well as synergistic activity with trastuzumab. In a pivotal phase III trial, a combination of lapatinib and capecitabine significantly decreased the risk of disease progression relative to capecitabine alone in women with HER2-positive advanced or metastatic breast cancer previously treated with anthracyclines, taxanes, and trastuzumab. Other trials are evaluating lapatinib in inflammatory breast cancer—for which encouraging data have been reported—in combination with hormone therapy, in combination with trastuzumab, and as an adjunct to adjuvant therapy for early-stage disease. Notably, lapatinib has not been associated with serious or symptomatic cardiotoxicity in clinical trials. It can cross the blood–brain barrier and might therefore have a role in preventing central-nervous-system progression. These features make lapatinib an ideal agent to evaluate more fully in HER2-positive metastatic and early-stage breast cancer.
Key Points
-
Lapatinib is a novel small molecule inhibitor of EGFR and HER2 with proven efficacy in HER2-positive breast cancer
-
Randomized phase III trial data showed that in women with advanced or metastatic breast cancer who received prior therapy with an anthracycline, a taxane, and trastuzumab in the metastatic setting, a combination of lapatinib plus capecitabine produced a 43% reduction in risk of disease progression compared with capecitabine alone
-
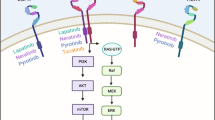
The efficacy of lapatinib in trastuzumab-resistant disease might be due to its different mechanism of action; lapatinib targets the internal tyrosine kinase domain of both EGFR and HER2 whereas trastuzumab targets the extracellular domain of HER2
-
These divergent mechanisms of action may also be responsible for the synergy demonstrated by the agents in preclinical studies
-
Lapatinib seems to have a favorable cardiac tolerability profile; the incidence of cardiac events was relatively low in the pivotal trial, and all decreases in LVEF were reversible and asymptomatic
-
Lapatinib is an ideal agent to study as an adjunct to adjuvant therapy for HER2-positive breast cancer because of its demonstrated activity in HER2-positive advanced breast cancer, its reasonable safety profile, ease of administration, and its ability to cross the blood–brain barrier
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Dixon RB et al. (2004) Chapter 33: Cancer of the Breast: Section 1: Molecular Biology of Breast Cancer. In Cancer: Principles and Practice of Oncology, edn 7 (Eds DeVita VT. et al.) Philadelphia: Lippincott Williams & Wilkins
Linggi B and Carpenter G (2006) ErbB receptors: new insights on mechanisms and biology. Trends Cell Biol 16: 649–656
Xia W et al. (2004) Truncated ErbB2 receptor (p95ErbB2) is regulated by heregulin through heterodimer formation with ErbB3 yet remains sensitive to the dual EGFR/ErbB2 kinase inhibitor GW572016. Oncogene 23: 646–653
Slamon DJ et al. (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344: 783–792
Cobleigh MA et al. (1999) Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol 17: 2639–2648
Vogel CL et al. (2002) Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol 20: 719–726
Romond EH et al. (2005) Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 353: 1673–1684
Smith I et al. for the HERA study team (2007) 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet 369: 29–36
Nahta R et al. (2006) Mechanisms of Disease: understanding resistance of HER2 therapy in human breast cancer. Nat Clin Pract Oncol 3: 269–280
Guarneri V et al. (2006) Long term cardiac tolerability of trastuzumab in metastatic breast cancer: the MD Anderson Cancer Center experience. J Clin Oncol 1: 4107–4115
Pugatsch T et al. (2006) Anti-erbB2 treatment induces cardiotoxicity by interfering with cell survival pathways. Breast Cancer Res 8: R35
Crone SA et al. (2002) ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat Med 8: 459–465
Tykerb® [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2007
Konecny GE et al. (2006) Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res 66: 1630–1639
Rusnak DW et al. (2001) The characterization of novel, dual ErbB-2/EGFR, tyrosine kinase inhibitors: potential therapy for cancer. Cancer Res 61: 7196–7203
Rusnak DW et al. (2001) The effects of the novel, reversible epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo. Mol Cancer Ther 1: 85–94
Nahta R et al. (2007) Lapatinib induces apoptosis in trastuzumab-resistant breast cancer cells: effects on insulin-like growth factor I signaling. Mol Cancer Ther 6: 667–674
Xia W et al. (2007) Lapatinib antitumor activity is not dependent upon phosphatase and tensin homologue deleted on chromosome 10 in ErbB2-overexpressing breast cancers. Cancer Res 67: 1170–1175
Xia W et al. (2005) Combining lapatinib (GW572016), a small molecule inhibitor of ErbB1 and ErbB2 tyrosine kinases, with therapeutic anti-ErbB2 antibodies enhances apoptosis of ErbB2-overexpressing breast cancer cells. Oncogene 24: 6213–6221
Perez E et al. (2006) Cardiac safety experience in 3558 patients treated with lapatinib [abstract #1420] Ann Oncol 17 (Suppl 9)
Bence AK et al. (2005) Phase I pharmacokinetic studies evaluating single and multiple doses of oral GW572016, a dual EGFR-ErbB2 inhibitor, in healthy subjects. Invest New Drugs 23: 39–49
Versola M et al. (2004) Clinical activity of GW572016 in EGF10003 in patients with solid tumors [abstract #3047]. J Clin Oncol 22 (Suppl)
Spector NL et al. (2005) Study of the biologic effects of lapatinib, a reversible inhibitor of ErbB1 and ErbB2 tyrosine kinases, on tumor growth and survival pathways in patients with advanced malignancies. J Clin Oncol 23: 2502–2512
Burris HA et al. (2005) Phase I safety, pharmacokinetics, and clinical activity study of lapatinib (GW572016), a reversible dual inhibitor of epidermal growth factor receptor tyrosine kinases, in heavily pretreated patients with metastatic carcinomas. J Clin Oncol 2: 5305–5313
Gomez HL et al. (2006) Results from a phase II randomized study of lapatinib as first-line treatment for patients with HER2-amplified locally advanced or metastatic breast cancer. [http://www.posters2view.com/sabcs06/view.php?nu=1090] (accessed 19 June 2007)
Blackwell KL et al. (2005) Determining relevant biomarkers from tissue and serum that may predict response to single agent lapatinib in trastuzumab refractory metastatic breast cancer [abstract #3004]. J Clin Oncol 23: 193s
Burstein H et al. (2004) A phase II, open-label, multicenter study of lapatinib in two cohorts of patients with advanced or metastatic breast cancer who have progressed while receiving trastuzumab-containing regimens [abstract #1040]. Ann Oncol 15 (Suppl 3): 27
Baselga J et al. (2005) Phase II study of efficacy, safety, and pharmacokinetics of trastuzumab monotherapy administered on a 3-weekly schedule. J Clin Oncol 23: 2162–2171
Clinicaltrials.gov (online 7 January 2004) Lapatinib in combination with trastuzumab versus lapatinib monotherapy in subjects with metastatic breast cancer [http://clinicaltrials.gov/ct/show/NCT00320385] (accessed 30 April 2007)
Clinicaltrials.gov. Paclitaxel with/without GW572016 (Lapatinib) as first line therapy for women with advanced or metastatic breast cancer [http://www.clinicaltrials.gov/ct/show/NCT00075270?order=1] (accessed 26 September 2007)
Clinicaltrials.gov. Study in women and men with metastatic breast cancer that have overexpression of ErbB2 [http://www.clinicaltrials.gov/ct/show/NCT00281658?order=1] (accessed 26 September 2007)
Clinicaltrials.gov. ErbB2 over-expressing metastatic breast cancer study using paclitaxel, trastuzumab, and lapatinib [http://www.clinicaltrials.gov/ct/show/NCT00272987?order=1] (accessed 26 September 2007)
Clinicaltrials.gov. Study comparing GW572016 and letrozole versus letrozole in subjects with advanced or metastatic breast cancer [http://clinicaltrials.gov/ct/show/NCT00073528?order=2] (accessed 19 June 2007)
Clinicaltrials.gov. Fulvestrant with or without lapatinib in treating postmenopausal women with stage III or stage IV breast cancer that is hormone receptor-positive and expresses HER2 [http://clinicaltrials.gov/show/NCT00390455] (accessed 30 April 2007)
Clinicaltrials.gov. Tykerb Evaluation After Chemotherapy (TEACH): lapatinib versus placebo in women with early-stage breast cancer [http://clinicaltrials.gov/ct/show/NCT00374322?order=1] (accessed 30 April 2007)
Clinicaltrials.gov. Adjuvant lapatinib and/or trastuzumab treatment optimisation study (ALTTO) [http://www.clinicaltrials.gov/ct/show/NCT00490139?order=1] (accessed 26 September 2007)
Geyer CE et al. (2006) Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 355: 2733–2743
De Bono J et al. (2003) Phase I and pharmacokinetic (PK) study of oral GW572016, a potent reversible dual inhibitor of both ErbB1 and ErbB2 tyrosine kinase (TK), administered in combination with capecitabine [abstract #901]. J Clin Oncol 21 (Suppl)
Schwartz G et al. (2004) Phase I clinical, biology & pharmacokinetic study of the combination of GW572016 and capecitabine in patients with advanced solid tumors [abstract #3070]. J Clin Oncol 22 (Suppl)
Cameron D et al. (2007) Lapatinib (L) plus capecitabine (C) in HER2+ advanced breast cancer (ABC): updated efficacy and biomarker analysis [abstract #1035]. J Clin Oncol 25 (Suppl)
Clayton AJ et al. (2004) Incidence of cerebral metastases in patients treated with trastuzumab for metastatic breast cancer. Br J Cancer 91: 639–643
Yau T et al. (2006) Incidence, pattern and timing of brain metastases among patients with advanced breast cancer treated with trastuzumab. Acta Oncol 45: 196–201
Lin NU et al. (2004) CNS metastases in breast cancer. J Clin Oncol 22: 3608–3617
Stemmler HJ et al. (2007) Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood-brain barrier. Anticancer Drugs 18: 23–28
Lin NU et al. (2006) Phase II trial of lapatinib for brain metastases in patients with HER2+ breast cancer [abstract #503]. J Clin Oncol 24 (Suppl)
Yang CH et al. (2005-2006) Systemic treatments for inflammatory breast cancer. Breast Dis 22: 55–65
Cristofanilli M et al. (2006) A phase II combination study of lapatinib and paclitaxel as a neoadjuvant therapy in patients with newly diagnosed inflammatory breast cancer (IBC) [abstract #1]. In Proceedings of the San Antonio Breast Cancer Symposium, 2006 December 14–17, San Antonio, TX
Trudeau M et al. (2006) Lapatinib (Tykerb) monotherapy in patients (pts) with recurrent inflammatory breast cancer (BC): clinical activity and biologic predictors of response [abstract #1400]. Ann Oncol 17 (Suppl 9): ix69
Clinicaltrials.gov. Docetaxel and lapatinib with or without combination chemotherapy or docetaxel and trastuzumab with combination chemotherapy in treating women with locally advanced, inflammatory, or resectable breast cancer [http://clinicaltrials.gov/ct/show/NCT00450892?order=1] (accessed 30 April 2007)
Storniolo A et al. (2005) A phase I, open-label study of lapatinib (GW572016) plus trastuzumab; a clinically active regimen [abstract #559]. J Clin Oncol 23: 18s
Chu I et al. (2005) The dual ErbB1/ErbB2 inhibitor, lapatinib (GW572016), cooperates with tamoxifen to inhibit both cell proliferation- and estrogen-dependent gene expression in antiestrogen-resistant breast cancer. Cancer Res 65: 18–25
Clinicaltrials.gov. Lapatinib and tamoxifen in treating patients with locally advanced or metastatic breast cancer that did not respond to previous tamoxifen [http://clinicaltrials.gov/ct/show/NCT00118157?order=2] (accessed 30 April 2007)
Chu Q et al. (2005) A phase I, open-label study of the safety, tolerability and pharmacokinetics of lapatinib (GW572016) in combination with letrozole in cancer patients [abstract #3001]. J Clin Oncol 23: 192s
Polychronis A et al. (2005) Preoperative gefitinib versus gefitinib and anastrozole in postmenopausal patients with oestrogen-receptor positive and epidermal-growth-factor-receptor-positive primary breast cancer: a double-blind placebo-controlled phase II randomised trial. Lancet Oncol 6: 383–391
Tuma RS (2007) Lapatinib moves forward in inflammatory and early HER2-positive breast cancer. J Natl Cancer Inst 99: 348–349
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
David A Cameron is on the speaker's bureau for GlaxoSmithKline and Roche, and receives grant/research support from GlaxoSmithKline. Steven Stein receives stock and is an employee of GlaxoSmithKline.
Rights and permissions
About this article
Cite this article
Cameron, D., Stein, S. Drug Insight: intracellular inhibitors of HER2—clinical development of lapatinib in breast cancer. Nat Rev Clin Oncol 5, 512–520 (2008). https://doi.org/10.1038/ncponc1156
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncponc1156
This article is cited by
-
Glucose-6-phosphate dehydrogenase blockade potentiates tyrosine kinase inhibitor effect on breast cancer cells through autophagy perturbation
Journal of Experimental & Clinical Cancer Research (2019)
-
In vivo near-infrared imaging of ErbB2 expressing breast tumors with dual-axes confocal endomicroscopy using a targeted peptide
Scientific Reports (2017)
-
Effect of trastuzumab on the micellization properties, endocytic pathways and antitumor activities of polyurethane-based drug delivery system
Chinese Journal of Polymer Science (2017)
-
Prolactinoma ErbB receptor expression and targeted therapy for aggressive tumors
Endocrine (2014)
-
The anti-erbB3 antibody MM-121/SAR256212 in combination with trastuzumab exerts potent antitumor activity against trastuzumab-resistant breast cancer cells
Molecular Cancer (2013)