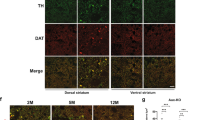
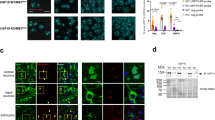
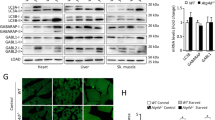
Abstract
Mice that are homozygous with respect to a mutation (axJ) in the ataxia (ax) gene develop severe tremors by 2–3 weeks of age followed by hindlimb paralysis and death by 6–10 weeks of age1. Here we show that ax encodes ubiquitin-specific protease 14 (Usp14). Ubiquitin proteases are a large family of cysteine proteases that specifically cleave ubiquitin conjugates. Although Usp14 can cleave a ubiquitin-tagged protein in vitro, it is unable to process polyubiquitin2, which is believed to be associated with the protein aggregates seen in Parkinson disease3, spinocerebellar ataxia type 1 (SCA1; ref. 4) and gracile axonal dystrophy (GAD)5. The physiological substrate of Usp14 may therefore contain a mono-ubiquitin side chain, the removal of which would regulate processes such as protein localization6 and protein activity7,8. Expression of Usp14 is significantly altered in axJ/axJ mice as a result of the insertion of an intracisternal-A particle (IAP) into intron 5 of Usp14. In contrast to other neurodegenerative disorders such as Parkinson disease and SCA1 in humans and GAD in mice, neither ubiquitin-positive protein aggregates nor neuronal cell loss is detectable in the central nervous system (CNS) of axJ mice. Instead, axJ mice have defects in synaptic transmission in both the central and peripheral nervous systems. These results suggest that ubiquitin proteases are important in regulating synaptic activity in mammals.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
MGD. Mouse Genomics Informatics Project (The Jackson Laboratory, Bar Harbor, Maine, 2001).
Yin, L., Krantz, B., Russell, N.S., Deshpande, S. & Wilkinson, K.D. Nonhydrolyzable diubiquitin analogues are inhibitors of ubiquitin conjugation and deconjugation. Biochemistry 39, 10001–10010 (2000).
McNaught, K.S., Olanow, C.W., Halliwell, B., Isacson, O. & Jenner, P. Failure of the ubiquitin–proteasome system in Parkinson's disease. Nat. Rev. Neurosci. 2, 589–594 (2001).
Skinner, P.J. et al. Ataxin-1 with an expanded glutamine tract alters nuclear matrix-associated structures. Nature 389, 971–974 (1997).
Saigoh, K. et al. Intragenic deletion in the gene encoding ubiquitin carboxy-terminal hydrolase in gad mice. Nat. Genet. 23, 47–51 (1999).
Katzmann, D.J., Babst, M. & Emr, S.D. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell 106, 145–155 (2001).
Hicke, L. Protein regulation by monoubiquitination. Nat. Rev. Mol. Cell Biol. 2, 195–201 (2001).
Shih, S.C., Sloper-Mould, K.E. & Hicke, L. Monoubiquitin carries a novel internalization signal that is appended to activated receptors. EMBO J. 19, 187–198 (2000).
D'Amato, C.J. & Hicks, S.P. Neuropathologic alterations in the ataxia (paralytic) mouse. Arch. Pathol. 80, 604–612 (1965).
Royaux, I., Bernier, B., Montgomery, J.C., Flaherty, L. & Goffinet, A.M. Reln(rl-Alb2), an allele of reeler isolated from a chlorambucil screen, is due to an IAP insertion with exon skipping. Genomics 42, 479–482 (1997).
Hamilton, B.A. et al. The vibrator mutation causes neurodegeneration via reduced expression of PITPα: positional complementation cloning and extragenic suppression. Neuron 18, 711–722 (1997).
Burt, A.M. Morphologic abnormalities in the postnatal differentiation of CA1 pyramidal cells and granule cells in the hippocampal formation of the ataxic mouse. Anat. Rec. 196, 61–69 (1980).
DiAntonio, A. et al. Ubiquitination-dependent mechanisms regulate synaptic growth and function. Nature 412, 449–452 (2001).
Sellin, L.C., Molgo, J., Tornquist, K., Hansson, B. & Thesleff, S. On the possible origin of giant or slow-rising miniature end-plate potentials at the neuromuscular junction. Pflugers Arch. 431, 325–334 (1996).
Wilson, D.F. Influence of presynaptic receptors on neuromuscular transmission in rat. Am. J. Physiol. 242, C366–C372 (1982).
Bhattacharyya, B.J. et al. Desensitization of mutant acetylcholine receptors in transgenic mice reduces the amplitude of neuromuscular synaptic currents. Synapse 27, 367–377 (1997).
Plomp, J.J. et al. Abnormal transmitter release at neuromuscular junctions of mice carrying the tottering α1A Ca2+ channel mutation. Brain 123, 463–471 (2000).
Washbourne, P. et al. Genetic ablation of the t-SNARE SNAP-25 distinguishes mechanisms of neuroexocytosis. Nat. Neurosci. 5, 19–26 (2002).
Duchen, L.W. & Strich, S.J. The effects of botulinum toxin on the pattern of innervation of skeletal muscle in the mouse. Q. J. Exp. Physiol. Cogn. Med. Sci. 53, 84–89 (1968).
Katz, B. & Thesleff, S. On the factors which determine the amplitude of the “miniature end plate potentials”. J. Physiol. 137, 267–278 (1957).
Katz, B. & Miledi, R. The role of calcium in neuromuscular facilitation. J. Physiol. 195, 481–492 (1968).
Matilla, A. et al. Mice lacking ataxin-1 display learning deficits and decreased hippocampal paired-pulse facilitation. J. Neurosci. 15, 5508–5516 (1998).
Silva, A.J. et al. Impaired learning in mice with abnormal short-lived plasticity. Curr. Biol. 1, 1509–1518 (1996).
Chain, D.G., Schwartz, J.H. & Hegde, A.N. Ubiquitin-mediated proteolysis in learning and memory. Mol. Neurobiol. 20, 125–142 (1999).
Rosahl, T.W. et al. Essential functions of synapsins I and II in synaptic vesicle regulation. Nature 375, 488–493 (1995).
Detera-Wadleigh, S.D. et al. A high-density genome scan detects evidence for a bipolar-disorder susceptibility locus on 13q32 and other potential loci on 1q32 and 18p11.2. Proc. Natl Acad. Sci. USA 96, 5604–5609 (1999).
Schwab, S.G. et al. Support for a chromosome 18p locus conferring susceptibility to functional psychoses in families with schizophrenia, by association and linkage analysis. Am. J. Hum. Genet. 63, 1139–1152 (1998).
Wilson, S.M. et al. Mutations in Cdh23 cause nonsyndromic hearing loss in waltzer mice. Genomics 74, 228–233 (2001).
Hollingsworth, E.B. et al. Biochemical characterization of a filtered synaptoneurosome preparation from guinea pig cerebral cortex: cyclic adenosine 3′:5′-monophosphate-generating systems, receptors, and enzymes. J. Neurosci. 5, 2240–2253 (1985).
Dionne, V.E. & Stevens, C.F. Voltage dependence of agonist effectiveness at the frog neuromuscular junction: resolution of a paradox. J. Physiol. 251, 245–270 (1975).
Acknowledgements
We thank L. Dobrunz and J. Wilson for critical reading of the manuscript; D. Swing, F. Dorsey, J. Dietz and R. Koogle for maintenance and care of the mice; D. Gilbert and T.N. O'Sullivan for technical assistance; K. Rogers, J. Matta and B. Smith for tissue sectioning; R. Frederickson for composition of the figures; and A. Borodovsky and H. Ploegh for providing the antibodies against Usp14. This work was supported by grants from the US National Institutes of Health (to R.J.M.) and the US National Cancer Institute and US Department of Health and Human Services (to N.G.C. and N.A.J.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Wilson, S., Bhattacharyya, B., Rachel, R. et al. Synaptic defects in ataxia mice result from a mutation in Usp14, encoding a ubiquitin-specific protease. Nat Genet 32, 420–425 (2002). https://doi.org/10.1038/ng1006
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng1006
This article is cited by
-
Deubiquitinating enzymes (DUBs): decipher underlying basis of neurodegenerative diseases
Molecular Psychiatry (2022)
-
Screening for differentially expressed circRNAs in ischemic stroke by RNA sequencing
BMC Neurology (2021)
-
Deubiquitylating enzymes in neuronal health and disease
Cell Death & Disease (2021)
-
Ubiquitin signalling in neurodegeneration: mechanisms and therapeutic opportunities
Cell Death & Differentiation (2021)
-
Deubiquitylating enzymes and drug discovery: emerging opportunities
Nature Reviews Drug Discovery (2018)