Abstract
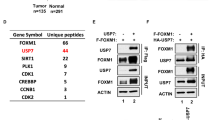
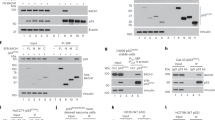
We have previously shown that ASPP1 and ASPP2 are specific activators of p53; one mechanism by which wild-type p53 is tolerated in human breast carcinomas is through loss of ASPP activity. We have further shown that 53BP2, which corresponds to a C-terminal fragment of ASPP2, acts as a dominant negative inhibitor of p53 (ref. 1). Hence, an inhibitory form of ASPP resembling 53BP2 could allow cells to bypass the tumor-suppressor functions of p53 and the ASPP proteins. Here, we characterize such a protein, iASPP (inhibitory member of the ASPP family), encoded by PPP1R13L in humans and ape-1 in Caenorhabditis elegans. iASPP is an evolutionarily conserved inhibitor of p53; inhibition of iASPP by RNA-mediated interference or antisense RNA in C. elegans or human cells, respectively, induces p53-dependent apoptosis. Moreover, iASPP is an oncoprotein that cooperates with Ras, E1A and E7, but not mutant p53, to transform cells in vitro. Increased expression of iASPP also confers resistance to ultraviolet radiation and to cisplatin-induced apoptosis. iASPP expression is upregulated in human breast carcinomas expressing wild-type p53 and normal levels of ASPP. Inhibition of iASPP could provide an important new strategy for treating tumors expressing wild-type p53.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Samuels-Lev, Y. et al. ASPP proteins specifically stimulate the apoptotic function of p53. Mol. Cell 8, 781–794 (2001).
Metzstein, M.M., Stanfield, G.M. & Horvitz, H.R. Genetics of programmed cell death in C. elegans: past, present and future. Trends Genet. 14, 410–416 (1998).
Derry, W.B., Putzke, A.P. & Rothman, J.H. Caenorhabditis elegans p53: role in apoptosis, meiosis and stress resistance. Science 294, 591–595 (2001).
Schumacher, B., Hofmann, K., Boulton, S. & Gartner, A. The C. elegans homolog of the p53 tumor suppressor is required for DNA damage–induced apoptosis. Curr. Biol. 11, 1722–1727 (2001).
Yang, J.-P., Hori, M., Sanda, T. & Okamoto, T. Identification of a novel inhibitor of nuclear factor-kB, RelA-associated inhibitor. J. Biol. Chem. 274, 15662–15670 (1999).
Gorina, S. & Pavletich, N.P. Structure of the p53 tumor suppressor bound to the ankyrin and SH3 domains of 53BP2. Science 274, 1001–1005 (1996).
Brodsky, M.H. et al. Drosophila p53 binds a damage-response element at the reaper locus. Cell 101, 103–113 (2000).
Ollmann, M. et al. Drosophila p53 is a structural and functional homolog of the tumor suppressor p53. Cell 101, 91–101 (2000).
Harlow, E.E. & Lane, D.P. Antibodies: A Laboratory Manual (Cold Spring Harbor Laboratory Press, New York, 1988).
Crook, T., Marston, N.J., Sara, E.A. & Vousden, K.H. Transcription activation by p53 correlates with suppression of growth but not transformation. Cell 79, 817–827 (1994).
Hsieh, J.-K., Fredersdorf, S., Kouzarides, T., Martin, K. & Lu, X. E2F1-induced apoptosis requires DNA binding but not transcriptional activity and is inhibited by the retinoblastoma protein through direct interaction. Genes Dev. 11, 1840–1852 (1997).
Fire, A. et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391, 806–811 (1998).
Acknowledgements
We would like to thank E. Slee and C. Stephens for reading the manuscript, S. Ali for technical help, the Human Genome Mapping Project Resource Centre for providing the EST clone of iASPP and The Caenorhabditis Genetics Center and the international C. elegans gene knockout consortium for providing the ced-3 and cep-1 mutants. This work was primarily funded by the Ludwig Institute for Cancer Research. D.B. is funded by Association of International Cancer Research, and P.E.K. is supported by a Medical Research Council Senior Fellowship and grants from the Biotechnology and Biological Science Research Council and Janssen Pharmaceutica N.V.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Bergamaschi, D., Samuels, Y., O'Neil, N. et al. iASPP oncoprotein is a key inhibitor of p53 conserved from worm to human. Nat Genet 33, 162–167 (2003). https://doi.org/10.1038/ng1070
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng1070
This article is cited by
-
Regulation of immunological tolerance by the p53-inhibitor iASPP
Cell Death & Disease (2023)
-
iASPP suppression mediates terminal UPR and improves BRAF-inhibitor sensitivity of colon cancers
Cell Death & Differentiation (2023)
-
p53 inhibitor iASPP is an unexpected suppressor of KRAS and inflammation-driven pancreatic cancer
Cell Death & Differentiation (2023)
-
An analysis of the role of HnRNP C dysregulation in cancers
Biomarker Research (2022)
-
Thioredoxin-1 regulates self-renewal and differentiation of murine hematopoietic stem cells through p53 tumor suppressor
Experimental Hematology & Oncology (2022)