Abstract
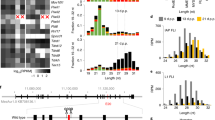
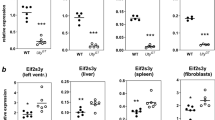
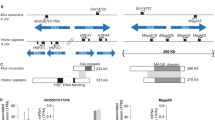
We identified the gene carrying the juvenile spermatogonial depletion mutation (jsd), a recessive spermatogenic defect mapped to mouse chromosome 1 (refs. 1,2). We localized jsd to a 272-kb region and resequenced this area to identify the underlying mutation: a frameshift that severely truncates the predicted protein product of a 2.3-kb genomic open reading frame. This gene, Utp14b, evidently arose through reverse transcription of an mRNA from an X-linked gene and integration of the resulting cDNA into an intron of an autosomal gene, whose promoter and 5′ untranslated exons are shared with Utp14b. To our knowledge, Utp14b is the first protein-coding retrogene to be linked to a recessive mammalian phenotype. The X-linked progenitor of Utp14b is the mammalian ortholog of yeast Utp14, which encodes a protein required for processing of pre-rRNA3 and hence for ribosome assembly. Our findings substantiate the hypothesis4 that mammalian spermatogenesis is supported by autosomal retrogenes that evolved from X-linked housekeeping genes to compensate for silencing of the X chromosome during male meiosis5,6,7. We find that Utp14b-like retrogenes arose independently and were conserved during evolution in at least four mammalian lineages. This recurrence implies a strong selective pressure, perhaps to enable ribosome assembly in male meiotic cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Beamer, W.G., Cunliffe-Beamer, T.L., Shultz, K.L., Langley, S.H. & Roderick, T.H. Juvenile spermatogonial depletion (jsd): a genetic defect of germ cell proliferation of male mice. Biol. Reprod. 38, 899–908 (1988).
Boettger-Tong, H. et al. Identification and sequencing the juvenile spermatogonial depletion critical interval on mouse chromosome 1 reveals the presence of eight candidate genes. Biochem. Biophys. Res. Commun. 288, 1129–1135 (2001).
Dragon, F. et al. A large nucleolar U3 ribonucleoprotein required for 18S ribosomal RNA biogenesis. Nature 417, 967–970 (2002).
McCarrey, J.R. & Thomas, K. Human testis-specific PGK gene lacks introns and possesses characteristics of a processed gene. Nature 326, 501–505 (1987).
Handel, M.A., Park, C. & Kot, M. Genetic control of sex-chromosome inactivation during male meiosis. Cytogenet. Cell. Genet. 66, 83–88 (1994).
Solari, A.J. The behavior of the XY pair in mammals. Int. Rev. Cytol. 38, 273–317 (1974).
Richler, C. et al. Splicing components are excluded from the transcriptionally inactive XY body in male meiotic nuclei. Mol. Biol. Cell 5, 1341–1352 (1994).
Boettger-Tong, H.L., Johnston, D.S., Russell, L.D., Griswold, M.D. & Bishop, C.E. Juvenile spermatogonial depletion (jsd) mutant seminiferous tubules are capable of supporting transplanted spermatogenesis. Biol. Reprod. 63, 1185–1191 (2000).
Ohta, H. et al. Defect in germ cells, not in supporting cells, is the cause of male infertility in the jsd mutant mouse: proliferation of spermatogonial stem cells without differentiation. Int. J. Androl. 24, 15–23 (2001).
Chen, H.H., Liu, T.Y., Li, H. & Choo, K.B. Use of a common promoter by two juxtaposed and intronless mouse early embryonic genes, Rnf33 and Rnf35: implications in zygotic gene expression. Genomics 80, 140–143 (2002).
Scanlan, M.J. et al. Humoral immunity to human breast cancer: antigen definition and quantitative analysis of mRNA expression. Cancer Immun. 1, 4 (2001).
Dahl, H.-H.M., Brown, R.M., Hutchison, W.M., Maragos, C. & Brown, G.K. A testis-specific form of the human pyruvate dehydrogenase E1α subunit is coded for by an intronless gene on chromosome 4. Genomics 8, 225–232 (1990).
Hendriksen, P.J.M. et al. Testis-specific expression of a functional retroposon encoding glucose-6-phosphate dehydrogenase in the mouse. Genomics 41, 350–359 (1997).
Dass, B. et al. The gene for a variant form of the polyadenylation protein CstF-64 is on chromosome 19 and is expressed in pachytene spermatocytes in mice. J. Biol. Chem. 276, 8044–8050 (2001).
Sargent, C.A., Young, C., Marsh, S., Ferguson-Smith, M.A. & Affara, N.A. The glycerol kinase gene family: structure of the Xp gene, and related intronless retroposons. Hum. Mol. Genet. 3, 1317–1324 (1994).
Elliott, D.J. et al. An evolutionarily conserved germ cell-specific hnRNP is encoded by a retrotransposed gene. Hum. Mol. Genet. 9, 2117–2124 (2000).
Sedlacek, Z. et al. Human and mouse XAP-5 and XAP-5-like (X5L) genes: identification of an ancient functional retroposon differentially expressed in testis. Genomics 61, 125–132 (1999).
Mardon, G. et al. Mouse Zfx protein is similar to Zfy-2: each contains an acidic activating domain and 13 zinc fingers. Mol. Cell. Biol. 10, 681–688 (1990).
Ashworth, A., Skene, B., Swift, S. & Lovell-Badge, R. Zfa is an expressed retroposon derived from an alternative transcript of the Zfx gene. EMBO J. 9, 1529–1534 (1990).
Boer, P.H., Adra, C.N., Lau, Y.F. & McBurney, M.W. The testis-specific phosphoglycerate kinase gene pgk-2 is a recruited retroposon. Mol. Cell. Biol. 7, 3107–3112 (1987).
Halford, S. et al. Characterization of a novel human opsin gene with wide tissue expression and identification of embedded and flanking genes on chromosome 1q43. Genomics 72, 203–208 (2001).
Uechi, T., Maeda, N., Tanaka, T. & Kenmochi, N. Functional second genes generated by retrotransposition of the X-linked ribosomal protein genes. Nucleic Acids Res. 30, 5369–5375 (2002).
Cremers, F.P. et al. An autosomal homologue of the choroideremia gene colocalizes with the Usher syndrome type II locus on the distal part of chromosome 1q. Hum. Mol. Genet. 1, 71–75 (1992).
Banks, K.G. et al. Retroposon compensatory mechanism hypothesis not supported: Zfa knockout mice are fertile. Genomics 82, 254–260 (2003).
Tohda, A. et al. Testosterone suppresses spermatogenesis in juvenile spermatogonial depletion (jsd) mice. Biol. Reprod. 65, 532–537 (2001).
Kojima, Y. et al. Cessation of spermatogenesis in juvenile spermatogonial depletion (jsd/jsd) mice. Int. J. Urol. 4, 500–507 (1997).
Nagase, T. et al. Prediction of the coding sequences of unidentified human genes. VI. The coding sequences of 80 new genes (KIAA0201-KIAA0280) deduced by analysis of cDNA clones from cell line KG-1 and brain. DNA Res. 3, 321–329, 341–354 (1996).
Vanin, E.F. Processed pseudogenes. Characteristics and evolution. Biochim. Biophys. Acta 782, 231–241 (1984).
Zhang, Z., Harrison, P.M., Liu, Y. & Gerstein, M. Millions of years of evolution preserved: a comprehensive catalog of the processed pseudogenes in the human genome. Genome Res. 13, 2541–2558 (2003).
Lander, E.S. et al. Initial sequencing and analysis of the human genome. Nature 409, 860–921 (2001).
Acknowledgements
We thank W. Beamer for providing C57BL/6J jsd/+ mice; the Genome Sequencing group at the Whitehead/MIT Center for Genome Research for BAC sequencing; B. Birren and E.S. Lander for support; and A. Bortvin, J. Alfoldi, J. Koubova, J. Lange, J. Potash, J. Saionz, J. Wang, K. Kleene and S. Rozen for comments on the manuscript. This work was supported by the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Genetic linkage mapping of jsd. (PDF 74 kb)
Supplementary Fig. 2
Gene maps of the regions of the mouse and human X chromosomes that contain the Utp14a/UTP14A gene. (PDF 42 kb)
Supplementary Fig. 3
Amino acid alignments of human and mouse proteins homologous to yeast Utp14. (PDF 225 kb)
Supplementary Fig. 4
Gene maps of the UTP14C insertion region on human chromosome 13 and the syntenic region of the mouse genome. (PDF 39 kb)
Supplementary Fig. 5
Phylogenetic tree of jsd sequences. (PDF 19 kb)
Supplementary Table 1
Novel genetic markers generated in the course of genetic and physical mapping of the jsd critical region. (XLS 17 kb)
Rights and permissions
About this article
Cite this article
Bradley, J., Baltus, A., Skaletsky, H. et al. An X-to-autosome retrogene is required for spermatogenesis in mice. Nat Genet 36, 872–876 (2004). https://doi.org/10.1038/ng1390
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng1390
This article is cited by
-
Excess of retrogene traffic in pig X chromosome
Genetica (2019)
-
Amphioxus SYCP1: a case of retrogene replacement and co-option of regulatory elements adjacent to the ParaHox cluster
Development Genes and Evolution (2018)
-
RNA-based gene duplication: mechanistic and evolutionary insights
Nature Reviews Genetics (2009)
-
The consequences of asynapsis for mammalian meiosis
Nature Reviews Genetics (2009)