Abstract
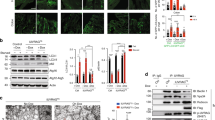
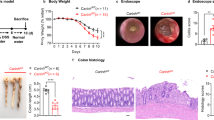
Leucine-rich repeat kinase 2 (LRRK2) has been identified by genome-wide association studies as being encoded by a major susceptibility gene for Crohn's disease. Here we found that LRRK2 deficiency conferred enhanced susceptibility to experimental colitis in mice. Mechanistic studies showed that LRRK2 was a potent negative regulator of the transcription factor NFAT and was a component of a complex that included the large noncoding RNA NRON (an NFAT repressor). Furthermore, the risk-associated allele encoding LRRK2 Met2397 identified by a genome-wide association study for Crohn's disease resulted in less LRRK2 protein post-translationally. Severe colitis in LRRK2-deficient mice was associated with enhanced nuclear localization of NFAT1. Thus, our study defines a new step in the control of NFAT activation that involves an immunoregulatory function of LRRK2 and has important implications for inflammatory bowel disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Abraham, C. & Cho, J.H. Inflammatory bowel disease. N. Engl. J. Med. 361, 2066–2078 (2009).
Barrett, J.C. et al. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn′s disease. Nat. Genet. 40, 955–962 (2008).
Franke, A. et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn′s disease susceptibility loci. Nat. Genet. 42, 1118–1125 (2010).
Anderson, C.A. et al. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat. Genet. 43, 246–252 (2011).
Kaser, A. & Blumberg, R.S. Endoplasmic reticulum stress in the intestinal epithelium and inflammatory bowel disease. Semin. Immunol. 21, 156–163 (2009).
Umeno, J. et al. Meta-analysis of published studies identified eight additional common susceptibility loci for Crohn′s disease and ulcerative colitis. Inflamm. Bowel Dis published online, doi:10.1002/ibd.21651 (23 February 2011).
Paisán-Ruíz, C. et al. Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron 44, 595–600 (2004).
Zimprich, A. et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 44, 601–607 (2004).
Gardet, A. et al. LRRK2 is involved in the IFN-γ response and host response to pathogens. J. Immunol. 185, 5577–5585 (2010).
Rao, A., Luo, C. & Hogan, P.G. Transcription factors of the NFAT family: regulation and function. Annu. Rev. Immunol. 15, 707–747 (1997).
Aliprantis, A.O. & Glimcher, L.H. NFATc1 in inflammatory and musculoskeletal conditions. Adv. Exp. Med. Biol. 658, 69–75 (2010).
Kao, S.C. et al. Calcineurin/NFAT signaling is required for neuregulin-regulated Schwann cell differentiation. Science 323, 651–654 (2009).
Graef, I.A. et al. Neurotrophins and netrins require calcineurin/NFAT signaling to stimulate outgrowth of embryonic axons. Cell 113, 657–670 (2003).
Horsley, V., Aliprantis, A.O., Polak, L., Glimcher, L.H. & Fuchs, E. NFATc1 balances quiescence and proliferation of skin stem cells. Cell 132, 299–310 (2008).
Rohini, A., Agrawal, N., Koyani, C.N. & Singh, R. Molecular targets and regulators of cardiac hypertrophy. Pharmacol. Res. 61, 269–280 (2010).
Ranger, A.M. et al. The transcription factor NF-ATc is essential for cardiac valve formation. Nature 392, 186–190 (1998).
Greenblatt, M.B., Aliprantis, A., Hu, B. & Glimcher, L.H. Calcineurin regulates innate antifungal immunity in neutrophils. J. Exp. Med. 207, 923–931 (2010).
Aramburu, J. et al. Regulation of the hypertonic stress response and other cellular functions by the Rel-like transcription factor NFAT5. Biochem. Pharmacol. 72, 1597–1604 (2006).
Hogan, P.G., Chen, L., Nardone, J. & Rao, A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 17, 2205–2232 (2003).
Willingham, A.T. et al. A strategy for probing the function of noncoding RNAs finds a repressor of NFAT. Science 309, 1570–1573 (2005).
Maekawa, T., Kubo, M., Yokoyama, I., Ohta, E. & Obata, F. Age-dependent and cell-population-restricted LRRK2 expression in normal mouse spleen. Biochem. Biophys. Res. Commun. 392, 431–435 (2010).
Tlaskalová-Hogenová, H. et al. Involvement of innate immunity in the development of inflammatory and autoimmune diseases. Ann. NY Acad. Sci. 1051, 787–798 (2005).
Berndt, B.E., Zhang, M., Chen, G.H., Huffnagle, G.B. & Kao, J.Y. The role of dendritic cells in the development of acute dextran sulfate sodium colitis. J. Immunol. 179, 6255–6262 (2007).
Ghia, J.E. et al. Role of M-CSF-dependent macrophages in colitis is driven by the nature of the inflammatory stimulus. Am. J. Physiol. Gastrointest. Liver Physiol. 294, G770–G777 (2008).
Ahrens, R. et al. Intestinal macrophage/epithelial cell-derived CCL11/eotaxin-1 mediates eosinophil recruitment and function in pediatric ulcerative colitis. J. Immunol. 181, 7390–7399 (2008).
Gwack, Y. et al. A genome-wide Drosophila RNAi screen identifies DYRK-family kinases as regulators of NFAT. Nature 441, 646–650 (2006).
Greggio, E. et al. The Parkinson disease-associated leucine-rich repeat kinase 2 (LRRK2) is a dimer that undergoes intramolecular autophosphorylation. J. Biol. Chem. 283, 16906–16914 (2008).
Shaw, K.T. et al. Immunosuppressive drugs prevent a rapid dephosphorylation of transcription factor NFAT1 in stimulated immune cells. Proc. Natl. Acad. Sci. USA 92, 11205–11209 (1995).
Monticelli, S. & Rao, A. NFAT1 and NFAT2 are positive regulators of IL-4 gene transcription. Eur. J. Immunol. 32, 2971–2978 (2002).
Zanoni, I. et al. CD14 regulates the dendritic cell life cycle after LPS exposure through NFAT activation. Nature 460, 264–268 (2009).
Goodridge, H.S., Simmons, R.M. & Underhill, D.M. Dectin-1 stimulation by Candida albicans yeast or zymosan triggers NFAT activation in macrophages and dendritic cells. J. Immunol. 178, 3107–3115 (2007).
Murthy, S.N. et al. Treatment of dextran sulfate sodium-induced murine colitis by intracolonic cyclosporin. Dig. Dis. Sci. 38, 1722–1734 (1993).
Melgar, S. et al. Validation of murine dextran sulfate sodium-induced colitis using four therapeutic agents for human inflammatory bowel disease. Int. Immunopharmacol. 8, 836–844 (2008).
Soriano-Izquierdo, A. et al. Effect of cyclosporin A on cell adhesion molecules and leukocyte-endothelial cell interactions in experimental colitis. Inflamm. Bowel Dis. 10, 789–800 (2004).
Satoh, Y. et al. Cyclosporine regulates intestinal epithelial apoptosis via TGF-β-related signaling. Am. J. Physiol. Gastrointest. Liver Physiol. 297, G514–G519 (2009).
West, A.B. et al. Parkinson's disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc. Natl. Acad. Sci. USA 102, 16842–16847 (2005).
Shih, T.C. et al. Aberrant activation of nuclear factor of activated T cell 2 in lamina propria mononuclear cells in ulcerative colitis. World J. Gastroenterol. 14, 1759–1767 (2008).
Weigmann, B. et al. The transcription factor NFATc2 controls IL-6-dependent T cell activation in experimental colitis. J. Exp. Med. 205, 2099–2110 (2008).
Good, M.C., Zalatan, J.G. & Lim, W.A. Scaffold proteins: hubs for controlling the flow of cellular information. Science 332, 680–686 (2011).
Parisiadou, L. & Cai, H. LRRK2 function on actin and microtubule dynamics in Parkinson disease. Commun. Integr. Biol. 3, 396–400 (2010).
Gehrke, S., Imai, Y., Sokol, N. & Lu, B. Pathogenic LRRK2 negatively regulates microRNA-mediated translational repression. Nature 466, 637–641 (2010).
Lin, X. et al. Leucine-rich repeat kinase 2 regulates the progression of neuropathology induced by Parkinson′s-disease-related mutant α-synuclein. Neuron 64, 807–827 (2009).
Tong, Y. et al. Loss of leucine-rich repeat kinase 2 causes impairment of protein degradation pathways, accumulation of α-synuclein, and apoptotic cell death in aged mice. Proc. Natl. Acad. Sci. USA 107, 9879–9884 (2010).
Alegre-Abarrategui, J. & Wade-Martins, R. Parkinson disease, LRRK2 and the endocytic-autophagic pathway. Autophagy 5, 1208–1210 (2009).
Plowey, E.D., Cherra, S.J. III, Liu, Y.J. & Chu, C.T. Role of autophagy in G2019S-LRRK2-associated neurite shortening in differentiated SH-SY5Y cells. J. Neurochem. 105, 1048–1056 (2008).
Altshuler, D., Daly, M.J. & Lander, E.S. Genetic mapping in human disease. Science 322, 881–888 (2008).
Wan, F. et al. Ribosomal protein S3: a KH domain subunit in NF-κB complexes that mediates selective gene regulation. Cell 131, 927–939 (2007).
Maxwell, J.R., Brown, A.W., Smith, C.L., Byrne, F.R. & Viney, J.L. Methods of inducing inflmmatory bowel disease in mice. Curr. Protoc. Pharmacol. 5, 1–37 (2009).
Acknowledgements
We thank M. Cookson (National Institute on Aging) for plasmid pCMV-2XMyc-LRRK2 expressing Myc-tagged wild-type or kinase-dead LRRK2; N. Bidere (Institut National de la Santé et de la Recherche Médicale) for luciferase constructs of NFAT and NF-κB and a renilla luciferase construct; N. Bidere, R. Germain, C. Kanellopoulou, B. Lo, A. Snow, J. Qin and H. Su for suggestions and comments; and colleagues at the Harvard Drosophila Screening Center for sharing screen results via the World Wide Web. Supported by the Intramural Research Program of the US National Institutes of Health, National Institute of Allergy and Infectious Diseases and National Institute on Aging.
Author information
Authors and Affiliations
Contributions
Z.L., J.L., S.K. and W.L. designed and did experiments; Z.L., H.C. and M.J.L. designed experiments and analyzed the data; and Z.L. and M.J.L. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–12 (PDF 3198 kb)
Rights and permissions
About this article
Cite this article
Liu, Z., Lee, J., Krummey, S. et al. The kinase LRRK2 is a regulator of the transcription factor NFAT that modulates the severity of inflammatory bowel disease. Nat Immunol 12, 1063–1070 (2011). https://doi.org/10.1038/ni.2113
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2113
This article is cited by
-
Mutant LRRK2 exacerbates immune response and neurodegeneration in a chronic model of experimental colitis
Acta Neuropathologica (2023)
-
Mutant LRRK2 in lymphocytes regulates neurodegeneration via IL-6 in an inflammatory model of Parkinson’s disease
npj Parkinson's Disease (2022)
-
Dapl1 controls NFATc2 activation to regulate CD8+ T cell exhaustion and responses in chronic infection and cancer
Nature Cell Biology (2022)
-
Effect of LRRK2 protein and activity on stimulated cytokines in human monocytes and macrophages
npj Parkinson's Disease (2022)
-
Association of LRRK2 rs11564258 single nucleotide polymorphisms with type and extent of gastrointestinal mycobiome in ulcerative colitis: a case–control study
Gut Pathogens (2021)