Abstract
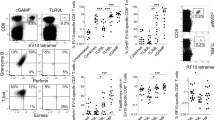
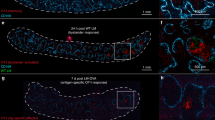
Naive T cells are stimulated by antigen-presenting dendritic cells (DCs) in secondary lymphoid organs, but whether other types of cell participate in T cell priming is unclear. Here we show in mice that natural killer (NK) cells, which are normally excluded from lymph nodes, are rapidly recruited in a CCR7-independent, CXCR3-dependent manner to lymph nodes on stimulation by the injection of mature DCs. Recruitment of NK cells is also induced by some, but not all, adjuvants and correlates with the induction of T helper cell type 1 (TH1) responses. NK cell depletion and reconstitution experiments show that NK cells provide an early source of interferon-γ (IFN-γ) that is necessary for TH1 polarization. Taken together, our results identify an induced pathway of NK cell migration in antigen-stimulated lymph nodes and a mechanism by which some adjuvants may facilitate TH1 responses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Banchereau, J. & Steinman, R.M. Dendritic cells and the control of immunity. Nature 392, 245–252 (1998).
Moingeon, P., Haensler, J. & Lindberg, A. Towards the rational design of Th1 adjuvants. Vaccine 19, 4363–4372 (2001).
Medzhitov, R. & Janeway, C.A. Jr. Innate immune induction of the adaptive immune response. Cold Spring Harb. Symp. Quant. Biol. 64, 429–435 (1999).
Rot, A. & von Andrian, U.H. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu. Rev. Immunol. 22, 891–928 (2004).
von Andrian, U.H. & Mempel, T.R. Homing and cellular traffic in lymph nodes. Nat. Rev. Immunol. 3, 867–878 (2003).
Forster, R. et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 99, 23–33 (1999).
Gunn, M.D. et al. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J. Exp. Med. 189, 451–460 (1999).
Sallusto, F., Geginat, J. & Lanzavecchia, A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 22, 745–763 (2004).
Scimone, M.L. et al. CXCL12 mediates CCR7-independent homing of central memory cells, but not naive T cells, in peripheral lymph nodes. J. Exp. Med. 199, 1113–1120 (2004).
Martín-Fontecha, A. et al. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J. Exp. Med. 198, 615–621 (2003).
Amsen, D. et al. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell 117, 515–526 (2004).
Macatonia, S.E. et al. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J. Immunol. 154, 5071–5079 (1995).
Cella, M. et al. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J. Exp. Med. 184, 747–752 (1996).
Snijders, A., Kalinski, P., Hilkens, C.M. & Kapsenberg, M.L. High-level IL-12 production by human dendritic cells requires two signals. Int. Immunol. 10, 1593–1598 (1998).
Afkarian, M. et al. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. Nat. Immunol. 3, 549–557 (2002).
Szabo, S.J., Sullivan, B.M., Peng, S.L. & Glimcher, L.H. Molecular mechanisms regulating Th1 immune responses. Annu. Rev. Immunol. 21, 713–758 (2003).
Moretta, L. et al. Human natural killer cells: their origin, receptors and function. Eur. J. Immunol. 32, 1205–1211 (2002).
Diefenbach, A. & Raulet, D.H. Innate immune recognition by stimulatory immunoreceptors. Curr. Opin. Immunol. 15, 37–44 (2003).
Cerwenka, A. & Lanier, L.L. Natural killer cells, viruses and cancer. Nat. Rev. Immunol. 1, 41–49 (2001).
Yokoyama, W.M., Kim, S. & French, A.R. The dynamic life of natural killer cells. Annu. Rev. Immunol. 22, 405–429 (2004).
Moretta, A. Natural killer cells and dendritic cells: rendezvous in abused tissues. Nat. Rev. Immunol. 2, 957–964 (2002).
Cooper, M.A., Fehniger, T.A., Fuchs, A., Colonna, M. & Caligiuri, M.A. NK cell and DC interactions. Trends Immunol. 25, 47–52 (2004).
Gerosa, F. et al. Reciprocal activating interaction between natural killer cells and dendritic cells. J. Exp. Med. 195, 327–333 (2002).
Ferlazzo, G. et al. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J. Exp. Med. 195, 343–351 (2002).
Piccioli, D., Sbrana, S., Melandri, E. & Valiante, N.M. Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J. Exp. Med. 195, 335–341 (2002).
Mailliard, R.B. et al. Dendritic cells mediate NK cell help for Th1 and CTL responses: two-signal requirement for the induction of NK cell helper function. J. Immunol. 171, 2366–2373 (2003).
Scharton, T.M. & Scott, P. Natural killer cells are a source of interferon γ that drives differentiation of CD4+ T cell subsets and induces early resistance to Leishmania major in mice. J. Exp. Med. 178, 567–577 (1993).
Fehniger, T.A. et al. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell–derived IL-2: a potential new link between adaptive and innate immunity. Blood 101, 3052–3057 (2003).
Ferlazzo, G. et al. The abundant NK cells in human secondary lymphoid tissues require activation to express killer cell Ig-like receptors and become cytolytic. J. Immunol. 172, 1455–1462 (2004).
Bjorkdahl, O. et al. Characterization of CC-chemokine receptor 7 expression on murine T cells in lymphoid tissues. Immunology 110, 170–179 (2003).
Hsieh, C.S., Macatonia, S.E., O'Garra, A. & Murphy, K.M. T cell genetic background determines default T helper phenotype development in vitro. J. Exp. Med. 181, 713–721 (1995).
Szabo, S.J., Dighe, A.S., Gubler, U. & Murphy, K.M. Regulation of the interleukin (IL)-12R β2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J. Exp. Med. 185, 817–824 (1997).
Alpan, O., Bachelder, E., Isil, E., Arnheiter, H. & Matzinger, P. 'Educated' dendritic cells act as messengers from memory to naive T helper cells. Nat. Immunol. 5, 615–622 (2004).
Frigerio, S. et al. β Cells are responsible for CXCR3-mediated T-cell infiltration in insulitis. Nat. Med. 8, 1414–1420 (2002).
Jiang, D. et al. Regulation of pulmonary fibrosis by chemokine receptor CXCR3. J. Clin. Invest. 114, 291–299 (2004).
Janatpour, M.J., Hudak, S., Sathe, M., Sedgwick, J.D. & McEvoy, L.M. Tumor necrosis factor–dependent segmental control of MIG expression by high endothelial venules in inflamed lymph nodes regulates monocyte recruitment. J. Exp. Med. 194, 1375–1384 (2001).
Yoneyama, H. et al. Evidence for recruitment of plasmacytoid dendritic cell precursors to inflamed lymph nodes through high endothelial venules. Int. Immunol. 16, 915–928 (2004).
Yoneyama, H. et al. Pivotal role of dendritic cell–derived CXCL10 in the retention of T helper cell 1 lymphocytes in secondary lymph nodes. J. Exp. Med. 195, 1257–1266 (2002).
Trinchieri, G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 3, 133–146 (2003).
Moser, M. & Murphy, K.M. Dendritic cell regulation of TH1-TH2 development. Nat. Immunol. 1, 199–205 (2000).
Snijders, A., Kalinski, P., Hilkens, C.M. & Kapsenberg, M.L. High-level IL-12 production by human dendritic cells requires two signals. Int. Immunol. 10, 1593–1598 (1998).
Grogan, J.L. et al. Early transcription and silencing of cytokine genes underlie polarization of T helper cell subsets. Immunity 14, 205–215 (2001).
Noben-Trauth, N., Hu-Li, J. & Paul, W.E. Conventional, naive CD4+ T cells provide an initial source of IL-4 during Th2 differentiation. J. Immunol. 165, 3620–3625 (2000).
Granucci, F. et al. A contribution of mouse dendritic cell–derived IL-2 for NK cell activation. J. Exp. Med. 200, 287–295 (2004).
Vitale, M. et al. The small subset of CD56brightCD16 natural killer cells is selectively responsible for both cell proliferation and interferon-γ production upon interaction with dendritic cells. Eur. J. Immunol. 34, 1715–1722 (2004).
Lanzavecchia, A. & Sallusto, F. Dynamics of T lymphocyte responses: intermediates, effectors, and memory cells. Science 290, 92–97 (2000).
Wakil, A.E., Wang, Z.E., Ryan, J.C., Fowell, D.J. & Locksley, R.M. Interferon γ derived from CD4+ T cells is sufficient to mediate T helper cell type 1 development. J. Exp. Med. 188, 1651–1656 (1998).
Satoskar, A.R. et al. Mice lacking NK cells develop an efficient Th1 response and control cutaneous Leishmania major infection. J. Immunol. 162, 6747–6754 (1999).
O'Hagan, D.T. & Valiante, N.M. Recent advances in the discovery and delivery of vaccine adjuvants. Nat. Rev. Drug Discov. 2, 727–735 (2003).
Hancock, W.W. et al. Requirement of the chemokine receptor CXCR3 for acute allograft rejection. J. Exp. Med. 192, 1515–1520 (2000).
Lutz, M.B. et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 223, 77–92 (1999).
Okada, T. et al. Chemokine requirements for B cell entry to lymph nodes and Peyer's patches. J. Exp. Med. 196, 65–75 (2002).
Acknowledgements
We thank M. Uguccioni for reading the manuscript and for comments. This work was supported by grants from the Swiss National Science Foundation (31-63885) and from the European Commission ('Memovax' QLK2-CT-2001-01205 and 'Main' FP6-502935). The Institute for Research in Biomedicine is supported by the Helmut Horten Foundation.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Kinetics of NK cell migration to lymph nodes. (PDF 62 kb)
Supplementary Fig. 2
Injection in vivo of anti-asialo GM-1 antibody results in the depletion of NK cells but not of αβ T cells, γδ T cells, or B cells. (PDF 116 kb)
Supplementary Fig. 3
Purity of NK cells used for transfer and migration experiments. (PDF 43 kb)
Supplementary Fig. 4
B6D2F1 mice were adoptively transferred with DO11.10 T cells and depleted of NK cells by injection of anti-NK1.1 antibody. (PDF 74 kb)
Rights and permissions
About this article
Cite this article
Martín-Fontecha, A., Thomsen, L., Brett, S. et al. Induced recruitment of NK cells to lymph nodes provides IFN-γ for TH1 priming. Nat Immunol 5, 1260–1265 (2004). https://doi.org/10.1038/ni1138
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1138
This article is cited by
-
The inter-link of ageing, cancer and immunity: findings from real-world retrospective study
Immunity & Ageing (2023)
-
Heterologous SARS-CoV-2 spike protein booster elicits durable and broad antibody responses against the receptor-binding domain
Nature Communications (2023)
-
Autologous dendritic cell vaccination against HIV-1 induces changes in natural killer cell phenotype and functionality
npj Vaccines (2023)
-
Innate lymphoid cells and innate-like T cells in cancer — at the crossroads of innate and adaptive immunity
Nature Reviews Cancer (2023)
-
Reciprocal expression of the immune response genes CXCR3 and IFI44L as module hubs are associated with patient survivals in primary central nervous system lymphoma
International Journal of Clinical Oncology (2023)