Abstract
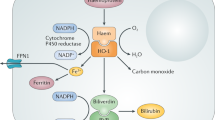
Chronic inflammatory diseases place a heavy social and economic burden on the resources of many nations, but the number of safe and effective treatments is limited. To date, the major research effort has concentrated on those mediators responsible for the initiation and maintenance of the pathological process. In contrast, little attention has been focused on endogenous factors responsible for the resolution of the inflammation. Heme oxygenase ((HO); EC 1.14.99.3) is the rate–limiting enzyme in the catabolism of heme to biliverdin (which is converted to bilirubin by biliverdin reductase), free iron and carbon monoxide (CO). Two isoforms of HO have been characterized, the constitutive isoform, HO–2, which is the major isoform present under physiological conditions, and the stress–induced isoform, HO–1, which has also been classified as heat–shock protein 32K (ref. 1). Increases in HO activity have been implicated in tissue protection against oxidative stress2. In this communication, we describe the effects of modulating HO during an acute complement–dependent inflammatory response. Elevation of this enzyme resulted in a striking suppression, whereas inhibition of the enzyme led to a potentiation of the inflammatory response. Such novel enzyme modulation has application on the one hand to the treatment of inflammatory diseases and on the other hand to immunosuppressed states in which the impaired ability to mount an adequate inflammatory response may result in death from opportunistic infections.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Maines, M.D. Heme oxygenase: Function, multiplicity, regulatory mechanisms, and clinical application. FASEB J. 2, 2557–2568 (1988).
Nath, K.A. et al. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J. Clin. Invest. 90, 267–270 (1992).
Capasso, F., Dunn, C.J., Yamamoto, S., Willoughby, D.A. & Giroud, R.A. Further studies on carrageenan-induced pleurisy in rats. J. Pathol. 116, 117–124 (1975).
Cantoni, L., Rossi, C., Rizzardini, M., Gadina, M. & Ghezzi, P. Interleukin-1 and tumor necrosis factor induce hepatic haem oxygenase. Biochem. J. 279, 891–894 (1991).
Keyse, S.M. & Tyrrell, R.M. Heme oxygenase is the major 32-kDa stress protein induced in the human skin fibroblasts by UV radiation, hydrogen peroxide and sodium arsenite. Proc. Natl. Acad. Sci. USA 86, 99–103 (1991).
Polla, B.S., Stubbe, H., Kantengwa, S., Maridonneau-Parini, I. & Jacquier-Sarlin, M.R. Differential induction of stress proteins and functional effects of heat shock in human phagocytes. Inflammation 19, 363–371 (1995).
Stacker, R., Yamamoto, Y. & McDonagh, A.F. Bilirubin is an antioxidant of possible physiological importance. Science 235, 1043–1046 (1987).
Nakagami, T., Toyomura, K., Kinoshita, T. & Morisawa, S.A. A beneficial role of bile pigments as an endogenous tissue protector: Anti-complement effects of biliverdin and conjugated bilirubin. Biochim. Biophys. Acta. 1158, 189–193 (1993).
Marks, G.S., Brien, J.F., Nakatsu, K. & McLaughlin, B.E. Does carbon monoxide have a physiological function? Trends Pharmacol. Sci. 12, 185–188 (1991).
Morita, T., Perrella, M.A., Lee, M. & Kourembanas, S. Smooth muscle Cell-derived carbon-monoxide is a regulator of vascular cGMP. Proc. Natl. Acad. Sci. USA 92, 1475–1479 (1995).
White, K.A. & Marietta, M.A. Nitric oxide synthase is a cytochrome P450 type hemoprotein. Biochemistry 31, 6627–6631 (1992).
Martasek, P., Schwartzman, M.L. & Goodman, A.I. Hemin and L-arginine regulation of blood pressure in spontaneous hypersensitive rats. J. Am. Soc. Nephrol. 2, 1078–1084 (1993).
Tomlinson, A. et al. Cyclo-oxygenase and nitric oxide synthase isoforms in rat carrageenin-induced pleurisy. Br. J. Pharmac. 113, 693–698 (1994).
Vane, J.R. et al. Inducible isoforms of cyclo-oxygenase and nitric oxide synthase in inflammation. Proc. Natl. Acad. Sci. USA 91, 2046–2050 (1994).
Willis, D., Tomlinson, A., Frederick, R., Paul-Clark, M.J. & Willoughby, D.A. Modulation of heme oxygenase activity in rat brain and spleen by inhibitors and donors of nitric oxide. Biochem. Biophys. Res. Comtn. 214, 1152–1156 (1995).
Levere, R.D. et al. Elevated levels of heme oxygenase-1 activity and mRNA in peripheral blood adherent cells of acquired immunodeficiency syndrome patients. Am. J. Hematol. 43, 19–23 (1993).
Sierra, E.S. & Nutter, L.M.A. A microassay for heme oxygenase activity using thin-layer chromatography. Anal. Biochem. 200, 27–30 (1992).
Bradford, M.M. A rapid and sensitive method for the quantisation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Willis, D., Moore, A., Frederick, R. et al. Heme oxygenase: A novel target for the modulation of inflammatory response. Nat Med 2, 87–90 (1996). https://doi.org/10.1038/nm0196-87
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nm0196-87
This article is cited by
-
Mesenchymal stem cells genetically engineered to express platelet-derived growth factor and heme oxygenase-1 ameliorate osteoarthritis in a canine model
Journal of Orthopaedic Surgery and Research (2021)
-
Pathogenic role of angiotensin II and the NF-κB system in a model of malignant hypertensive nephrosclerosis
Hypertension Research (2019)
-
Protective effects of chebulic acid from Terminalia chebula Retz. against t-BHP-induced oxidative stress by modulations of Nrf2 and its related enzymes in HepG2 cells
Food Science and Biotechnology (2019)
-
Bronchiectasis
Nature Reviews Disease Primers (2018)
-
Homocysteine Induces Glial Reactivity in Adult Rat Astrocyte Cultures
Molecular Neurobiology (2018)