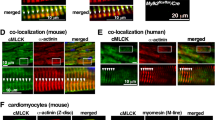
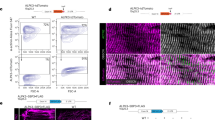
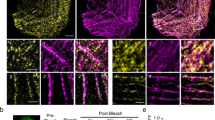
Abstract
Signaling by the calcium-dependent phosphatase calcineurin profoundly influences the growth and gene expression of cardiac and skeletal muscle. Calcineurin binds to calsarcins, a family of muscle-specific proteins of the sarcomeric Z-disc, a focal point in the pathogenesis of human cardiomyopathies. We show that calsarcin-1 negatively modulates the functions of calcineurin, such that calcineurin signaling was enhanced in striated muscles of mice that do not express calsarcin-1. As a consequence of inappropriate calcineurin activation, mice with a null mutation in calsarcin-1 showed an excess of slow skeletal muscle fibers. The absence of calsarcin-1 also activated a hypertrophic gene program, despite the absence of hypertrophy, and enhanced the cardiac growth response to pressure overload. In contrast, cardiac adaptation to other hypertrophic stimuli, such as chronic catecholamine stimulation or exercise, was not affected. These findings show important roles for calsarcins as modulators of calcineurin signaling and the transmission of a specific subset of stress signals leading to cardiac remodeling in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Olson, E.N. & Williams, R.S. Calcineurin signaling and muscle remodeling. Cell 101, 689–692 (2000).
Frey, N., McKinsey, T.A. & Olson, E.N. Decoding calcium signals involved in cardiac growth and function. Nat. Med. 6, 1221–1227 (2000).
Crabtree, G.R. & Olson, E.N. NFAT signaling: choreographing the social lives of cells. Cell 109 Suppl, 67–79 (2002).
Hogan, P.G., Chen, L., Nardone, J. & Rao, A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 17, 2205–2232 (2003).
Molkentin, J.D. et al. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 93, 215–228 (1998).
Sussman, M.A. et al. Pathogenesis of dilated cardiomyopathy: molecular, structural, and population analyses in tropomodulin-overexpressing transgenic mice. Am. J. Pathol. 155, 2101–2113 (1999).
Wang, Y. et al. Direct biomechanical induction of endogenous calcineurin inhibitor Down Syndrome Critical Region-1 in cardiac myocytes. Am. J. Physiol. Heart Circ. Physiol. 283, H533–H539 (2002).
Hill, J.A. et al. Cardiac hypertrophy is not a required compensatory response to short-term pressure overload. Circulation 101, 2863–2869 (2000).
Rothermel, B.A. et al. Myocyte-enriched calcineurin-interacting protein, MCIP1, inhibits cardiac hypertrophy in vivo. Proc. Natl. Acad. Sci. USA 98, 3328–3333 (2001).
Hill, J.A. et al. Targeted inhibition of calcineurin in pressure-overload cardiac hypertrophy. Preservation of systolic function. J. Biol. Chem. 277, 10251–10255 (2002).
Taigen, T., De Windt, L.J., Lim, H.W. & Molkentin, J.D. Targeted inhibition of calcineurin prevents agonist-induced cardiomyocyte hypertrophy. Proc. Natl. Acad. Sci. USA 97, 1196–1201 (2000).
Serrano, A.L. et al. Calcineurin controls nerve activity-dependent specification of slow skeletal muscle fibers but not muscle growth. Proc. Natl. Acad. Sci. USA 98, 13108–13113 (2001).
Naya, F.J. et al. Stimulation of slow skeletal muscle fiber gene expression by calcineurin in vivo. J. Biol. Chem. 275, 4545–4548 (2000).
Chin, E.R. et al. A calcineurin-dependent transcriptional pathway controls skeletal muscle fiber type. Genes Dev. 12, 2499–2509 (1998).
Frey, N., Richardson, J.A. & Olson, E.N. Calsarcins, a novel family of sarcomeric calcineurin-binding proteins. Proc. Natl. Acad. Sci. USA 97, 14632–14637 (2000).
Frey, N. & Olson, E.N. Calsarcin-3, a novel skeletal muscle-specific member of the calsarcin family, interacts with multiple Z-disc proteins. J. Biol. Chem. 277, 13998–14004 (2002).
Faulkner, G. et al. FATZ, a filamin-, actinin-, and telethonin-binding protein of the Z-disc of skeletal muscle. J. Biol. Chem. 275, 41234–41242 (2000).
Takada, F. et al. Myozenin: an alpha-actinin- and gamma-filamin-binding protein of skeletal muscle Z lines. Proc. Natl. Acad. Sci. USA 98, 1595–1600 (2001).
Knoll, R. et al. The cardiac mechanical stretch sensor machinery involves a Z disc complex that is defective in a subset of human dilated cardiomyopathy. Cell 111, 943–955 (2002).
Kovacic-Milivojevic, B. et al. Focal adhesion kinase and p130Cas mediate both sarcomeric organization and activation of genes associated with cardiac myocyte hypertrophy. Mol. Biol. Cell 12, 2290–2307 (2001).
Furukawa, T. et al. Specific interaction of the potassium channel beta-subunit minK with the sarcomeric protein T-cap suggests a T-tubule-myofibril linking system. J. Mol. Biol. 313, 775–784 (2001).
Berchtold, M.W., Brinkmeier, H. & Muntener, M. Calcium ion in skeletal muscle: its crucial role for muscle function, plasticity, and disease. Physiol. Rev. 80, 1215–1265 (2000).
Schiaffino, S. & Serrano, A. Calcineurin signaling and neural control of skeletal muscle fiber type and size. Trends Pharmacol. Sci. 23, 569–575 (2002).
Parsons, S.A., Wilkins, B.J., Bueno, O.F. & Molkentin, J.D. Altered skeletal muscle phenotypes in calcineurin Aalpha and Abeta gene-targeted mice. Mol. Cell. Biol. 23, 4331–4343 (2003).
Yang, J. et al. Independent signals control expression of the calcineurin inhibitory proteins MCIP1 and MCIP2 in striated muscles. Circ. Res. 87, E61–E68 (2000).
Lim, H.W. et al. Calcineurin expression, activation, and function in cardiac pressure-overload hypertrophy. Circulation 101, 2431–2437 (2000).
Antos, C.L. et al. Activated glycogen synthase-3 beta suppresses cardiac hypertrophy in vivo. Proc. Natl. Acad. Sci. USA 99, 907–912 (2002).
Brancaccio, M. et al. Melusin, a muscle-specific integrin beta1-interacting protein, is required to prevent cardiac failure in response to chronic pressure overload. Nat. Med. 9, 68–75 (2003).
Saito, S. et al. beta-Adrenergic pathway induces apoptosis through calcineurin activation in cardiac myocytes. J. Biol. Chem. 275, 34528–34533 (2000).
De Windt, L.J. et al. Targeted inhibition of calcineurin attenuates cardiac hypertrophy in vivo. Proc. Natl. Acad. Sci. USA 98, 3322–3327 (2001).
Morisco, C. et al. The Akt-glycogen synthase kinase 3beta pathway regulates transcription of atrial natriuretic factor induced by beta-adrenergic receptor stimulation in cardiac myocytes. J. Biol. Chem. 275, 14466–14475 (2000).
Ryeom, S., Greenwald, R.J., Sharpe, A.H. & McKeon, F. The threshold pattern of calcineurin-dependent gene expression is altered by loss of the endogenous inhibitor calcipressin. Nat. Immunol. 4, 874–881 (2003).
Hilioti, Z. et al. GSK-3 kinases enhance calcineurin signaling by phosphorylation of RCNs. Genes Dev. 18, 35–47 (2004).
Vega, R.B., Yang, J., Rothermel, B.A., Bassel-Duby, R. & Williams, R.S. Multiple domains of MCIP1 contribute to inhibition of calcineurin activity. J. Biol. Chem. 277, 30401–30407 (2002).
Chang, S. et al. Histone deacetylases 5 and 9 govern responsiveness of the heart to a subset of stress signals and play redundant roles in heart development. Mol. Cell. Biol. 24, 8467–8476 (2004).
Zhang, C.L. et al. Class II histone deacetylases act as signal-responsive repressors of cardiac hypertrophy. Cell 110, 479–488 (2002).
Liu, Y., Cseresnyes, Z., Randall, W.R. & Schneider, M.F. Activity-dependent nuclear translocation and intranuclear distribution of NFATc in adult skeletal muscle fibers. J. Cell Biol. 155, 27–39 (2001).
Sah, R. et al. Inhibition of calcineurin and sarcolemmal Ca2+ influx protects cardiac morphology and ventricular function in K(v)4.2N transgenic mice. Circulation 105, 1850–1856 (2002).
Chien, K.R. Genomic circuits and the integrative biology of cardiac diseases. Nature 407, 227–232 (2000).
Clark, K.A., McElhinny, A.S., Beckerle, M.C. & Gregorio, C.C. Striated muscle cytoarchitecture: an intricate web of form and function. Annu. Rev. Cell Dev. Biol. 18, 637–706 (2002).
Schonberger, J. & Seidman, C.E. Many roads lead to a broken heart: the genetics of dilated cardiomyopathy. Am. J. Hum. Genet. 69, 249–260 (2001).
Mohapatra, B. et al. Mutations in the muscle LIM protein and alpha-actinin-2 genes in dilated cardiomyopathy and endocardial fibroelastosis. Mol. Genet. Metab. 80, 207–215 (2003).
Vatta, M. et al. Mutations in Cypher/ZASP in patients with dilated cardiomyopathy and left ventricular non-compaction. J. Am. Coll. Cardiol. 42, 2014–2027 (2003).
Arimura, T. et al. A Cypher/ZASP mutation associated with dilated cardiomyopathy alters the binding affinity to protein kinase C. J. Biol. Chem. 279, 6746–6752 (2003).
Heineke, J. et al. Downregulation of cytoskeletal muscle LIM protein by nitric oxide: impact on cardiac myocyte hypertrophy. Circulation 107, 1424–1432 (2003).
Wu, H. et al. Activation of MEF2 by muscle activity is mediated through a calcineurin-dependent pathway. EMBO J. 20, 6414–6423 (2001).
Wettschureck, N. et al. Absence of pressure overload induced myocardial hypertrophy after conditional inactivation of Gq/G11 . Nat. Med. 7, 1236–1240 (2001).
Acknowledgements
We thank A. Tizenor for graphics, J. Page for editorial assistance, and U. Öhl and J. Emma for excellent technical assistance. D. J. Garry provided the running cages for mouse exercise training. The advice of T. Fehm on statistical analyses is appreciated. We are grateful to S. Schiaffino and E. Bush for providing antibodies and H. Weiler (Blood Research Institute) for ES cell targeting. E.N.O. was supported by grants from the US National Institutes of Health, the Donald W. Reynolds Foundation, the Robert A. Welch Foundation and the Texas Advanced Technology Program. T.B was supported by the US National Institutes of Health minority supplement grant. N.F. was supported by grants of the Deutsche Forschungsgemeinschaft (Fr1289/1-1 and Fr1289/3-1) and the Young Investigator Program of the University of Heidelberg.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Smaller cardiomyocytes in calsarcin-deficient hearts. (PDF 92 kb)
Supplementary Fig. 2
Calsarcin inhibits calcineurin activity in vitro. (PDF 37 kb)
Supplementary Fig. 3
Calsarcin-1 null/MCIP-transgenic mice display superinduction of ANF-expression. (PDF 97 kb)
Supplementary Fig. 4
Similar degree of exercise-induced hypertrophy in wild-type and calsarcin mutant hearts (PDF 45 kb)
Supplementary Table 1
Transthoracic echocardiographic analysis of wild-type and calsarcin mutant hearts (PDF 112 kb)
Supplementary Table 2
Characterization of left ventricular mechanics by pressure-volume measurements (PDF 143 kb)
Supplementary Table 3
Sequences of primers used in dot-blot and rea-time PCR experiments (PDF 113 kb)
Rights and permissions
About this article
Cite this article
Frey, N., Barrientos, T., Shelton, J. et al. Mice lacking calsarcin-1 are sensitized to calcineurin signaling and show accelerated cardiomyopathy in response to pathological biomechanical stress. Nat Med 10, 1336–1343 (2004). https://doi.org/10.1038/nm1132
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1132
This article is cited by
-
A missense mutation in the RSRSP stretch of Rbm20 causes dilated cardiomyopathy and atrial fibrillation in mice
Scientific Reports (2020)
-
MLP-deficient human pluripotent stem cell derived cardiomyocytes develop hypertrophic cardiomyopathy and heart failure phenotypes due to abnormal calcium handling
Cell Death & Disease (2019)
-
Hypertrophy of human embryonic stem cell–derived cardiomyocytes supported by positive feedback between Ca2+ and diacylglycerol signals
Pflügers Archiv - European Journal of Physiology (2019)
-
Progesterone signalling in broiler skeletal muscle is associated with divergent feed efficiency
BMC Systems Biology (2017)
-
G9a inhibits MEF2C activity to control sarcomere assembly
Scientific Reports (2016)