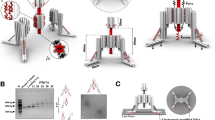
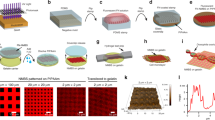
Abstract
Fibronectin, like other proteins involved in mechanotransduction, has the ability to exhibit recognition sites under mechanical stretch1,2,3. Such cryptic sites are buried inside the protein structure in the native fold and become exposed under an applied force4, thereby activating specific signalling pathways5. Here, we report the design of new active polymeric nanoassembled surfaces that show some similarities to these cryptic sites. These nanoassemblies consist of a first polyelectrolyte multilayer6 stratum loaded with enzymes and capped with a second polyelectrolyte multilayer acting as a mechanically sensitive nanobarrier. The biocatalytic activity of the film is switched on/off reversibly by mechanical stretching, which exposes enzymes through the capping barrier, similarly to mechanisms involved in proteins during mechanotransduction. This first example of a new class of biologically inspired surfaces should have great potential in the design of various devices aimed to trigger and modulate chemical reactions by mechanical action with applications in the field of microfluidic devices or mechanically controlled biopatches for example.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Vakonakis, I. D. S., Rooney, L. M. & Campbell, I. D. Interdomain association in fibronectin: Insight into cryptic sites and fibrillogenesis. EMBO J. 26, 2575–2583 (2007).
Smith, M. L. et al. Force-induced unfolding of fibronectin in the extracellular matrix of living cells. PLos Biol. 5, 2243–2254 (2007).
Gao, M. et al. Structure and functional significance of mechanically unfolded fibronectin type III1 intermediates. Proc. Natl Acad. Sci. USA 100, 14784–14789 (2003).
Vogel, V. & Sheetz, M. Local force and geometry sensing regulate cell functions. Nature Rev. Mol. Cell. Biol. 7, 265–275 (2006).
Vogel, V. Mechanotransduction involving multimodular proteins: Converting force into biochemical signals. Annu. Rev. Biophys. Biomol. Struct. 35, 459–488 (2006).
Decher, G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science 277, 1232–1237 (1997).
Hiller, J., Mendelsohn, J. D. & Rubner, M. F. Reversibly erasable nanoporous anti-reflection coatings from polyelectrolyte multilayers. Nature Mater. 1, 59–63 (2002).
Jiang, C. Y., Markutsya, S., Pikus, Y. & Tsukruk, V. V. Freely suspended nanocomposite membranes as highly sensitive sensors. Nature Mater. 3, 721–728 (2004).
Podsiadlo, P. et al. Ultrastrong and stiff layered polymer nanocomposites. Science 318, 80–83 (2007).
Lee, J. S. et al. Layer-by-layer assembled charge-trap memory devices with adjustable electronic properties. Nature Nanotech. 2, 790–795 (2007).
Tang, Z. Y., Wang, Y., Podsiadlo, P. & Kotov, N. A. Biomedical applications of layer-by-layer assembly: From biomimetics to tissue engineering. Adv. Mater. 18, 3203–3224 (2006).
Wood, K. C., Chuang, H. F., Batten, R. D., Lynn, D. M. & Hammond, P. T. Controlling interlayer diffusion to achieve sustained, multiagent delivery from layer-by-layer thin films. Proc. Natl Acad. Sci. USA 103, 10207–10212 (2006).
Picart, C. et al. Molecular basis for the explanation of the exponential growth of polyelectrolyte multilayers. Proc. Natl Acad. Sci. USA 99, 12531–12535 (2002).
Garza, J. M. et al. Multicompartment films made of alternate polyelectrolyte multilayers of exponential and linear growth. Langmuir 20, 7298–7302 (2004).
Mertz, D. et al. Mechanically responding nanovalves based on polyelectrolyte multilayers. Nano Lett. 7, 657–662 (2007).
Lu, C. H., Moehwald, H. & Fery, A. A lithography-free method for directed colloidal crystal assembly based on wrinkling. Soft Matter 3, 1530–1536 (2007).
Shutava, T. G., Kommireddy, D. S. & Lvov, Y. M. Layer-by-layer enzyme/polyelectrolyte films as a functional protective barrier in oxidizing media. J. Am. Chem. Soc. 128, 9926–9934 (2006).
Acknowledgements
Confocal microscopy was carried out in Strasbourg Esplanade Cellular Imaging Facility, funded by CNRS, INSERM, Louis Pasteur University and Alsace Region. We thank K. Benmlih for the build-up of the stretching devices and we are grateful to S. Lesko (Veeco, Dourdan, France) for helping with AFM experiments, D. Vautier and J. H. Lignot for fruitful discussions about immunogold detection, C. Ringwald for designing silicon cells and C. Bouthier for her assistance. We acknowledge support from the Région Alsace for financial contribution to the AFM equipment and P.S. thanks COST D43 for financial support.
Author information
Authors and Affiliations
Contributions
D.M., P.L. and C.V. carried out the experiments. P.S. and P.L. brought-up the concept. P.S. P.L. and J.C.V. designed the experiments. J.H. designed the experimental set-up. D.M., J.M. and C.V. analysed the data. V.B. proposed the adapted enzymatic model. P.S. and P.L. co-wrote the paper.
Corresponding author
Supplementary information
Supplementary Information
Supplementary Information (PDF 554 kb)
Rights and permissions
About this article
Cite this article
Mertz, D., Vogt, C., Hemmerlé, J. et al. Mechanotransductive surfaces for reversible biocatalysis activation. Nature Mater 8, 731–735 (2009). https://doi.org/10.1038/nmat2504
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat2504
This article is cited by
-
Shape morphing of hydrogels by harnessing enzyme enabled mechanoresponse
Nature Communications (2024)
-
Nano-frictional mechano-reinforcing porous nanowires scaffolds
Friction (2024)
-
The increasing dynamic, functional complexity of bio-interface materials
Nature Reviews Chemistry (2018)
-
Living biointerfaces based on non-pathogenic bacteria support stem cell differentiation
Scientific Reports (2016)
-
What are the emerging concepts and challenges in NANO? Nanoarchitectonics, hand-operating nanotechnology and mechanobiology
Polymer Journal (2016)