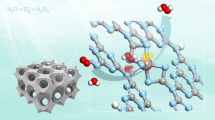
Abstract
The photocatalytic reduction of N2 to NH3 is typically hampered by poor binding of N2 to catalytic materials and by the very high energy of the intermediates involved in this reaction. Solvated electrons directly introduced into the reactant solution can provide an alternative pathway to overcome such limitations. Here we demonstrate that illuminated hydrogen-terminated diamond yields facile electron emission into water, thus inducing reduction of N2 to NH3 at ambient temperature and pressure. Transient absorption measurements at 632 nm reveal the presence of solvated electrons adjacent to the diamond after photoexcitation. Experiments using inexpensive synthetic diamond samples and diamond powder show that photocatalytic activity is strongly dependent on the surface termination and correlates with the production of solvated electrons. The use of diamond to eject electrons into a reactant liquid represents a new paradigm for photocatalytic reduction, bringing electrons directly to reactants without requiring molecular adsorption to the surface.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Swain, G. M. & Ramesham, R. The electrochemical activity of boron-doped polycrystalline diamond thin film electrodes. Anal. Chem. 65, 345–351 (1993).
Martin, H. B., Argoitia, A., Landau, U., Anderson, A. B. & Angus, J. C. Hydrogen and oxygen evolution on boron-doped diamond electrodes. J. Electrochem. Soc. 143, L133–L136 (1996).
Himpsel, F. J., Knapp, J. A., Vanvechten, J. A. & Eastman, D. E. Quantum photoyield of diamond(111) - stable negative-affinity emitter. Phys. Rev. B 20, 624–627 (1979).
Takeuchi, D. et al. Direct observation of negative electron affinity in hydrogen-terminated diamond surfaces. Appl. Phys. Lett. 86, 823–825 (2005).
Van der Weide, J. et al. Negative-electron-affinity effects on the diamond (100) surface. Phys. Rev. B 50, 5803–5806 (1994).
Bandis, C. & Pate, B. Photoelectric emission from negative-electron-affinity diamond (111) surfaces: Exciton breakup versus conduction-band emission. Phys. Rev. B 52, 12056–12071 (1995).
Bandis, C. & Pate, B. Electron emission due to exciton breakup from negative electron affinity diamond. Phys. Rev. Lett. 74, 777–780 (1995).
Takeuchi, D., Nebel, C. E. & Yamasaki, S. Surface defect states analysis on diamond by photoelectron emission yield experiments. Diamond Relat. Mater. 16, 823–825 (2007).
Ristein, J., Stein, W. & Ley, L. Defect spectroscopy and determination of the electron diffusion length in single crystal diamond by total photoelectron yield spectroscopy. Phys. Rev. Lett. 78, 1803–1806 (1997).
Pleskov, Y. V. in Diamond and Diamond-Like Film Applications (eds Giellise, P. J., Ivanov-Omskii, V. I., Popovici, G. & Prelas, M.) (Technomic, 1998).
Boonma, L., Yano, T., Tryk, D., Hashimoto, K. & Fujishima, A. Observation of photocurrent from band-gap excitation of semiconducting p-type diamond thin film electrodes. J. Electrochem. Soc. 144, L142–L145 (1997).
Krohn, C. E., Antoniewicz, P. R. & Thompson, J. C. Energetics for photoemission of electrons into NH3 and H2O. Surf. Sci. 101, 241–250 (1980).
Trasatti, S. The absolute electrode potential: An explanatory note. Pure Appl. Chem. 58, 955–966 (1986).
Jang, D. M. et al. Nanodiamonds as photocatalysts for reduction of water and graphene oxide. Chem. Commun. 48, 696–698 (2012).
Tennakone, K., Wickramanayake, S., Fernando, C. A. N., Ileperuma, O. A. & Punchihewa, S. Photocatalytic nitrogen reduction using visible light. J. Chem. Soc.; Chem. Commun. 14, 1078–1080 (1987).
Vettraino, M., Trudeau, M., Lo, A., Schurko, R. & Antonelli, D. Room-temperature ammonia formation from dinitrogen on a reduced mesoporous titanium oxide surface with metallic properties. J. Am. Chem. Soc. 124, 9567–9573 (2002).
Schrauzer, G. N. & Guth, T. D. Photocatalytic reactions. 1. Photolysis of water and photoreduction of nitrogen on titanium dioxide. J. Am. Chem. Soc. 99, 7189–7193 (1977).
Miyama, H., Fujii, N. & Nagae, Y. Heterogeneous photocatalytic synthesis of ammonia from water and nitrogen. Chem. Phys. Lett. 74, 523–524 (1980).
Leigh, G. J. Chemistry: Fixing nitrogen any which way. Science 279, 506–507 (1998).
Ranjit, K., Varadarajan, T. & Viswanathan, B. Photocatalytic reduction of dinitrogen to ammonia over noble-metal-loaded TiO2 . J. Photochem. Photobiol. A 96, 181–185 (1996).
Rao, N., Dube, S. & Natarajan, P. Photocatalytic reduction of nitrogen over (Fe, Ru or Os)/TiO2 catalysts. Appl. Cat. B 5, 33–42 (1994).
Bauer, N. Theoretical pathways for the reduction of N2 molecules in aqueous media: thermohynamics of N2Hn . J. Phys. Chem. 64, 833–837 (1960).
Bazhenova, T. & Shilov, A. Nitrogen fixation in solution. Coord. Chem. Rev. 144, 69–145 (1995).
Shilov, A. Catalytic reduction of molecular nitrogen in solutions. Russ. Chem. Bull. 53, 2555–2562 (2003).
Yandulov, D. V. & Schrock, R. R. Catalytic reduction of dinitrogen to ammonia at a single molybdenum center. Science 301, 76–78 (2003).
Ertl, G. Surface science and catalysis: Studies on the mechanism of ammonia synthesis. Catal Rev. Sci. Engr. 21, 201–223 (1980).
Rayment, T., Schlogl, R., Thomas, J. M. & Ertl, G. Structure of the ammonia-synthesis catalyst. Nature 315, 311–313 (1985).
Alpatova, N., Krishtalik, L. & Pleskov, Y. Electrochemistry of solvated electrons. Top. Curr. Chem. 138, 149–219 (1987).
Roduner, E. Hydrophobic solvation, quantum nature, and diffusion of atomic hydrogen in liquid water. Radiat. Phys. Chem. 72, 201–206 (2005).
Michael, B., Hart, E. & Schmidt, K. The absorption spectrum of eaq− in the temperature range −4 to 390. J. Phys. Chem. 144, 69–145 (1971).
Pálfi, T., Wojnárovits, L. & Takács, E. Calculated and measured transient product yields in pulse radiolysis of aqueous solutions: Concentration dependence. Radiat. Phys. Chem. 79, 1154–1158 (2010).
Hart, E. J. & Boag, J. W. Absorption spectrum of the hydrated electron in water and in aqueous solutions. J. Am. Chem. Soc. 84, 4090–4095 (1962).
Nakken, K. & Pihl, A. On the ability of nitrous oxide to convert hydrated electrons into hydroxyl radical. Radiat. Res. 26, 519–526 (1965).
Hoshino, K., Kuchii, R. & Ogawa, T. Dinitrogen photofixation properties of different titanium oxides in conducting polymer/titanium oxide hybrid systems. Appl. Cat. B 79, 81–88 (2008).
Litter, M. & Navio, J. Photocatalytic properties of iron-doped titania semiconductors. J. Photochem. Photobiol. A 98, 171–181 (1996).
Soria, J. et al. Dinitrogen photoreduction to ammonia over titanium dioxide powders doped with ferric ions. J. Phys. Chem. 95, 274–282 (1991).
Mozzanega, H., Herrmann, J. M. & Pichat, P. Ammonia oxidation over UV-irradiated titanium dioxide at room temperature. J. Phys. Chem. 83, 2251–2255 (1979).
Li, Q., Domen, K., Naito, S., Onishi, T. & Tamaru, K. Photocatalytic synthesis and photodecomposition of ammonia over SrTiO3 and BaTiO3 based catalysts. Chem. Lett. 3, 321–324 (1983).
Fujishima, A. & Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 238, 37–38 (1972).
Vouagner, D., Show, Y., Kiraly, B., Champagnon, B. & Girardeau-Montaut, J. P. Photoemission characteristics of diamond films. Appl. Surf. Sci. 168, 79–84 (2000).
Cui, J., Ristein, J. & Ley, L. Low-threshold electron emission from diamond. Phys. Rev. B 60, 16135–16142 (1999).
Baumann, P. K. & Nemanich, R. J. Surface cleaning, electronic states and electron affinity of diamond (100), (111) and (110) surfaces. Surf. Sci. 409, 320–335 (1998).
Baumann, P. & Nemanich, R. Electron affinity and Schottky barrier height of metal diamond (100),(111), and (110) interfaces. J. Appl. Phys. 83, 2072–2082 (1998).
Boucher, D. L., Davies, J. A., Edwards, J. G. & Mennad, A. An investigation of the putative photosynthesis of ammonia on iron-doped titania and other metal oxides. J. Photochem. Photobiol. 88, 53–64 (1995).
The HITRAN molecular spectroscopic database and HAWKS (HITRAN Atmospheric Workstation): 1996 Edition. J. Quant. Spectrosc. Radiat. Transf. 111, 1568–1613 (2010).
Hwang, D-Y. & Mebel, A. M. Reaction mechanism of N2/H2 conversion to NH3: A theoretical study. J. Phys. Chem. A 107, 2865–2874 (2003).
Siegbahn, P. & Westerberg, J. Nitrogen fixation by nitrogenases: a quantum chemical study. The J. Phys. Chem. B 102, 1615–1623 (1998).
Thoms, B. D., Owens, M. S., Butler, J. E. & Spiro, C. Production and characterization of smooth, hydrogen terminated diamond C(100). Appl. Phys. Lett. 65, 2957–2959 (1994).
Bolt, D. F. Colorimetric Determination of Nonmetals, Vol. 2 (Wiley, 1978).
Acknowledgements
The authors acknowledge L. C. Hamers, S. Hogendoorn, B. Putans and N. Becknell for their assistance with early stages of this work. The authors would also like to thank J. R. Schmidt, J. Christianson and G. Nathanson for useful insights into the N2 reduction mechanisms. This work was supported by the National Science Foundation DMR-1207281. Initial exploratory experiments were obtained through support from National Science Foundation DMR-1121288.
Author information
Authors and Affiliations
Contributions
R.J.H. designed and supervised the project, D.Z. carried out the experiments and wrote the paper, L.Z. assisted with infrared isotope labelling studies, and R.E.R. provided expertise in ultraviolet photoemission studies. All of the co-authors contributed to discussion and analysis of the data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 696 kb)
Rights and permissions
About this article
Cite this article
Zhu, D., Zhang, L., Ruther, R. et al. Photo-illuminated diamond as a solid-state source of solvated electrons in water for nitrogen reduction. Nature Mater 12, 836–841 (2013). https://doi.org/10.1038/nmat3696
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat3696
This article is cited by
-
Efficient electrosynthesis of formamide from carbon monoxide and nitrite on a Ru-dispersed Cu nanocluster catalyst
Nature Communications (2023)
-
Direct synthesis of hydrazine by efficient electrochemical ruthenium-catalysed ammonia oxidation
Nature Catalysis (2023)
-
Hydrogen-assisted activation of N2 molecules on atomic steps of ZnSe nanorods
Nano Research (2023)
-
Coupling Fe and Mo single atoms on hierarchical N-doped carbon nanotubes enhances electrochemical nitrogen reduction reaction performance
Nano Research (2023)
-
Preparation of Sulfur and Nitrogen Co-doped Carbon-Based Porous Nanomaterials for Efficient Electrocatalytic N2 Reduction
Journal of Electronic Materials (2023)