Abstract
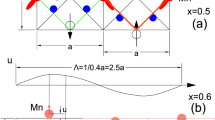
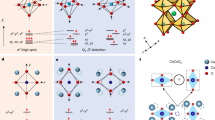
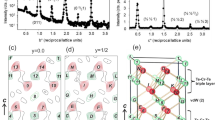
The cooperative Jahn–Teller effect (CJTE) refers to the correlation of distortions arising from individual Jahn–Teller centres in complex compounds1,2. The effect usually induces strong coupling between the static or dynamic charge, orbital and magnetic ordering, which has been related to many important phenomena such as colossal magnetoresistance1,3 and superconductivity1,4. Here we report a Na5/8MnO2 superstructure with a pronounced static CJTE that is coupled to an unusual Na vacancy ordering. We visualize this coupled distortion and Na ordering down to the atomic scale. The Mn planes are periodically distorted by a charge modulation on the Mn stripes, which in turn drives an unusually large displacement of some Na ions through long-ranged Na–O–Mn3+–O–Na interactions into a highly distorted octahedral site. At lower temperatures, magnetic order appears, in which Mn atomic stripes with different magnetic couplings are interwoven with each other. Our work demonstrates the strong interaction between alkali ordering, displacement, and electronic and magnetic structure, and underlines the important role that structural details play in determining electronic behaviour.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Bersuker, B. I. The Jahn–Teller Effect 1–616 (Cambridge Univ. Press, 2006).
Goodenough, J. Jahn–Teller phenomena in solids. Annu. Rev. Mater. Sci. 28, 1–27 (1998).
Raveau, B., Hervieu, M., Maignan, A. & Martin, C. The route to CMR manganites: what about charge ordering and phase separation? J. Mater. Chem. 11, 29–36 (2001).
Han, J. E., Gunnarsson, O. & Crespi, V. H. Strong superconductivity with local Jahn–Teller phonons in C60 solids. Phys. Rev. Lett. 90, 167006 (2003).
Berthelot, R., Carlier, D. & Delmas, C. Electrochemical investigation of the P2–NaxCoO2 phase diagram. Nature Mater. 10, 74–80 (2011).
Guignard, M. et al. P2-NaxVO2 system as electrodes for batteries and electron-correlated materials. Nature Mater. 12, 74–80 (2013).
Yabuuchi, N. et al. P2-type Nax[Fe1/2Mn1/2]O2 made from earth-abundant elements for rechargeable Na batteries. Nature Mater. 11, 512–517 (2012).
Roger, M. et al. Patterning of sodium ions and the control of electrons in sodium cobaltate. Nature 445, 631–634 (2007).
Zandbergen, H. W., Foo, M., Xu, Q., Kumar, V. & Cava, R. J. Sodium ion ordering in NaxCoO2: Electron diffraction study. Phys. Rev. B 70, 024101 (2004).
Huang, F-T. et al. Scanning transmission electron microscopy using selective high-order Laue zones: Three-dimensional atomic ordering in sodium cobaltate. Phys. Rev. Lett. 105, 125502 (2010).
Stock, C. et al. One-dimensional magnetic fluctuations in the spin-2 triangular lattice α-NaMnO2 . Phys. Rev. Lett. 103, 077202 (2009).
Wu, F., Yu, G., Xu, D. & Kan, E. First-principles investigations on the magnetic structure of α-NaMnO2 . J. Phys. Condens. Matter 24, 456002 (2012).
Giot, M. et al. Magnetoelastic coupling and symmetry breaking in the frustrated antiferromagnet α-NaMnO2 . Phys. Rev. Lett. 99, 247211 (2007).
Masquelier, C. et al. Chemical and magnetic characterization of spinel materials in the LiMn2O4–Li2 Mn4O9–Li4Mn5O12 system. J. Solid State Chem. 123, 255–266 (1996).
Borisevich, A. Y. et al. Suppression of octahedral tilts and associated changes in electronic properties at epitaxial oxide heterostructure interfaces. Phys. Rev. Lett. 105, 087204 (2010).
Jia, C. L. et al. Oxygen octahedron reconstruction in the SrTiO3/LaAlO3 heterointerfaces investigated using aberration-corrected ultrahigh-resolution transmission electron microscopy. Phys. Rev. B 79, 081405(R) (2009).
Barrier, N., Lebedev, O. I., Seikh, M. M., Porcher, F. & Raveau, B. Impact of Mn3+ upon structure and magnetism of the perovskite derivative Pb2−xBaxFeMnO5 (x ~ 0.7) . Inorg. Chem. 52, 6073–6082 (2013).
Ma, X., Chen, H. & Ceder, G. Electrochemical properties of monoclinic NaMnO2 . J. Electrochem. Soc. 158, A1307 (2011).
Tarascon, J. M. et al. In situ structural and electrochemical study of Ni1−xCoxO2 metastable oxides prepared by soft chemistry. J. Solid State Chem. 147, 410–420 (1999).
Delmas, C., Fouassier, C. & Hagenmuller, P. Structural classification and properties of the layered oxides. Phys. B+C99, 81–85 (1980).
Arroyo y de Dompablo, M. E., Van der Ven, A. & Ceder, G. First-principles calculations of lithium ordering and phase stability on LixNiO2 . Phys. Rev. B 66, 064112 (2002).
Peres, J., Weill, F. & Delmas, C. Lithium vacancy ordering in the monoclinic LixNiO2(0.50 < x < 0.75) solid solution. Solid State Ion. 116, 19–27 (1999).
Kubota, K., Kaneko, T., Yabuuchi, N., Hara, R. & Komaba, S. Electrode performance of lithium containing layered sodium iron manganese oxides for rechargeable Na-ion batteries. 224th ECS Meeting. Sodium-ion Batteries—Cathodes 1 (2013)
Lu, Z., MacNeil, D. D. & Dahn, J. R. Layered cathode materials Li[NixLi1/3−2x/3Mn2/3−x/3]O2 for lithium-ion batteries. Electrochem. Solid-State Lett. 4, A191–A194 (2001).
Chappert, C., Fert, A. & Van Dau, F. N. The emergence of spin electronics in data storage. Nature Mater. 6, 813–823 (2007).
Vojta, M. Lattice symmetry breaking in cuprate superconductors: stripes, nematics, and superconductivity. Adv. Phys. 58, 699–820 (2009).
Lee, S., Oshima, Y., Hosono, E., Zhou, H. & Takayanagi, K. Reversible contrast in focus series of annular bright field images of a crystalline LiMn2O4 nanowire. Ultramicroscopy 125, 43–48 (2013).
Lynn, J. W. et al. Double-focusing thermal triple-axis spectrometer at the NCNR. J. Res. NIST 117, 61–79 (2012).
Shirane, G., Shapiro, S. M. & Tranquada, J. M. Neutron Scattering with a Triple-Axis Spectrometer: Basic Techniques (Cambridge Univ. Press, 2002).
Ong, S. P. et al. Python Materials Genomics (pymatgen): A robust, open-source python library for materials analysis. Comput. Mater. Sci. 68, 314–319 (2013).
Acknowledgements
This work was supported by the Samsung Advanced Institute of Technology. The STEM work carried out at the Center for Functional Nanomaterials, Brookhaven National Laboratory (BNL), was supported by the US Department of Energy (DOE), Office of Basic Energy Sciences, under Contract No. DE-AC02-98CH10886. The neutron scattering activities by Y.S.L. were supported by DOE under Grant No. DE-FG02-07ER46134. Use of the National Synchrotron Light Source, BNL, was supported by the DOE, Office of Science, Office of Basic Energy Sciences, and by DOE-EERE under the Batteries for Advanced Transportation Technologies (BATT) Program, under Contract No. DE-AC02-98CH10886. The identification of commercial product or trade name does not imply recommendation by the National Institute of Standards and Technology. This work made use of the Shared Experimental Facilities supported in part by the MRSEC Program of the National Science Foundation under award number DMR-0819762. This work was also supported by a user project of ORNL’s Center for Nanophase Materials Sciences (CNMS), which is sponsored by the Scientific User Facilities Division, Office of Basic Energy Sciences, US Department of Energy. We appreciate the assistance with magnetic SQUID measurements from S. Chu at MIT.
Author information
Authors and Affiliations
Contributions
G.C. planned the study and supervised all aspects of the research. X.L. and G.C. wrote the manuscript. X.L. performed the electron diffraction measurement and DFT calculation, and solved the Na vacancy superstructure. X.L. calculated the spin exchange parameters by DFT and predicted the magnetic structure. X.M. and Y.L. synthesized the pristine compound. X.M. and X.L. obtained the superstructure phase by electrochemistry. X.L. and D.S. performed the STEM measurement and analysed together with J.C.I. the STEM results. L.L. and X.L. synthesized the superstructure phase by chemical de-intercalation. J.W.L. took the magnetic neutron diffraction data and order parameter, and R.C. and Y.S.L. solved the magnetic structure. X.L. took the magnetic susceptibility data. S.P.O. and A.T. calculated the phase diagram by DFT. J.B. and F.W. took the synchrotron XRD data. H.C., J.B. and X.L. performed the synchrotron XRD refinement. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 3271 kb)
Rights and permissions
About this article
Cite this article
Li, X., Ma, X., Su, D. et al. Direct visualization of the Jahn–Teller effect coupled to Na ordering in Na5/8MnO2. Nature Mater 13, 586–592 (2014). https://doi.org/10.1038/nmat3964
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat3964
This article is cited by
-
Mn-based cathode materials for rechargeable batteries
Science China Chemistry (2024)
-
α/β–Type NaMn0.89Ni0.11O2: as high-performance sodium-ion battery cathode
Ionics (2023)
-
The Effect of Nickel Salt on OMS-2 Nanorods in Oxygen Reduction Reaction
Catalysis Letters (2023)
-
Superior diffusion kinetics and electrochemical properties of α/β–type NaMn0.89Co0.11O2 as cathode for sodium-ion batteries
Journal of Solid State Electrochemistry (2022)
-
Review on Mn-based and Fe-based layered cathode materials for sodium-ion batteries
Ionics (2022)