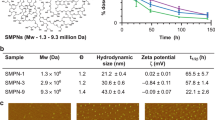
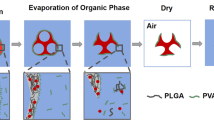
Abstract
Many nanosized particulate systems are being developed as intravascular carriers to increase the levels of therapeutic agents delivered to targets, with the fewest side effects1,2. The surface of these carriers is often functionalized with biological recognition molecules for specific, targeted delivery. However, there are a series of biological barriers in the body3,4,5 that prevent these carriers from localizing at their targets at sufficiently high therapeutic concentrations5,6. Here we show a multistage delivery system that can carry, release over time and deliver two types of nanoparticles into primary endothelial cells. The multistage delivery system is based on biodegradable and biocompatible mesoporous silicon particles that have well-controlled shapes, sizes and pores. The use of this system is envisioned to open new avenues for avoiding biological barriers and delivering more than one therapeutic agent to the target at a time, in a time-controlled fashion.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Brannon-Peppas, L. & Blanchette, J. O. Nanoparticle and targeted systems for cancer therapy. Adv. Drug Deliv. Rev. 56, 1649–1659 (2004).
Yezhelyev, M. V. et al. Emerging use of nanoparticles in diagnosis and treatment of breast cancer. Lancet Oncol. 7, 657–667 (2006).
Ferrari, M. Nanovector therapeutics. Curr. Opin. Chem. Biol. 9, 343–346 (2005).
Sakamoto, J., Annapragada, A., Decuzzi, P. & Ferrari, M. Antibiological barrier nanovector technology for cancer applications. Expert Opin. Drug Deliv. 4, 359–369 (2007).
Ferrari, M. Cancer nanotechnology: opportunities and challenges. Nature Rev. Cancer 5, 161–171 (2005).
Eckelman, W. C. & Mathis, C. A. Targeting proteins in vivo: In vitro guidelines. Nucl. Med. Biol. 33, 161–164 (2006).
Lin, M. Z., Teitell, M. A. & Schiller, G. J. The evolution of antibodies into versatile tumor-targeting agents. Clin. Cancer Res. 11, 129–138 (2005).
Farokhzad, O. C., Karp, J. M. & Langer, R. Nanoparticle–aptamer bioconjugates for cancer targeting. Expert Opin. Drug Deliv. 3, 311–324 (2006).
Torchilin, V. P. Multifunctional nanocarriers. Adv. Drug Deliv. Rev. 58, 1532–1555 (2006).
Shuvaev, V. V. et al. Factors modulating the delivery and effect of enzymatic cargo conjugated with antibodies targeted to the pulmonary endothelium. J. Controlled Release 118, 235–244 (2007).
Medina, O. P., Zhu, Y. & Kairemo, K. Targeted liposomal drug delivery in cancer. Curr. Pharm. Des. 10, 2981–2989 (2004).
Pierres, A., Benoliel, A.-M., Zhu, C. & Bongrand, P. Diffusion of microspheres in shear flow near a wall: use to measure binding rates between attached molecules. Biophys. J. 81, 25–42 (2001).
Decuzzi, P., Lee, S., Bhushan, B. & Ferrari, M. A theoretical model for the margination of particles within blood vessels. Ann. Biomed. Eng. 33, 179–190 (2005).
Decuzzi, P. F. & Ferrari, M. Fantastic voyages. Mech. Eng. 128, 24–27 (2006).
Decuzzi, P., Lee, S., Decuzzi, M. & Ferrari, M. Adhesion of microfabricated particles on vascular endothelium: a parametric analysis. Ann. Biomed. Eng. 32, 793–802 (2004).
Canham, L. T. et al. Derivatized mesoporous silicon with dramatically improved stability in simulated human blood plasma. Adv. Mater. 11, 1505–1507 (1999).
Bayliss, S. C., Heald, R., Fletcher, D. I. & Buckberry, L. D. The culture of mammalian cells on nanostructured silicon. Adv. Mater. 11, 318–321 (1999).
Chin, V., Collins, B. E., Sailor, M. J. & Bhatia, S. N. Compatibility of primary hepatocytes with oxidized nanoporous silicon. Adv. Mater. 13, 1877–1880 (2001).
Canham, L. T. Bioactive silicon structure fabrication through nanoetching techniques. Adv. Mater. 7, 1033–1037 (1995).
Foraker, A. B. et al. Microfabricated porous silicon particles enhance paracellular delivery of insulin across intestinal caco-2 cell monolayers. Pharm. Res. 20, 110–116 (2003).
Salonen, J. et al. Mesoporous silicon microparticles for oral drug delivery: Loading and release of five model drugs. J. Controlled Release 108, 362–374 (2005).
Thomas, J. C., Pacholski, C. & Sailor, M. J. Delivery of nanogram payloads using magnetic porous silicon microcarriers. Lab Chip 6, 782–787 (2006).
Stewart, M. P. & Buriak, J. M. Chemical and biological applications of porous silicon technology. Adv. Mater. 12, 859–869 (2000).
Song, J. H. & Sailor, M. J. Chemical modification of crystalline porous silicon surfaces. Comments Inorg. Chem. 21, 69–84 (1999).
Wang, Y. & Ferrari, M. Surface modification of micromachined silicon filters. J. Mater. Sci. 35, 4923–4930 (2000).
Decuzzi, P. & Ferrari, M. The adhesive strength of non-spherical particles mediated by specific interactions. Biomaterials 27, 5307–5314 (2006).
Lasic, D. D., Martin, F. J., Gabizon, A., Huang, S. K. & Papahadjopoulos, D. Sterically stabilized liposomes: a hypothesis on the molecular origin of the extended circulation times. Biochim. Biophys. Acta. 1070, 187–192 (1991).
Hirsch, L. R. et al. Nanoshell-mediated near-infrared thermal therapy of tumours under magnetic resonance guidance. Proc. Natl Acad. Sci. USA 100, 13549–13554 (2003).
Akin, D. et al. Bacteria-mediated delivery of nanoparticles and cargo into cells. Nature Nanotechn. 2, 441–449 (2007).
Kim, S. et al. Near-infrared fluorescent type II quantum dots for sentinel lymph node mapping. Nature Biotechnol. 22, 93–97 (2004).
Corot, C., Robert, P., Idee, J.-M. & Port, M. Recent advances in iron oxide nanocrystal technology for medical imaging. Adv. Drug Deliv. Rev. 58, 1471–1504 (2006).
Kam, N. W. S., O'Connell, M., Wisdom, J. A. & Dai, H. Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction. Proc. Natl Acad. Sci. USA 102, 11600–11605 (2005).
Acknowledgements
We thank the University of Texas at Austin for the use of the semiconductor cleanroom facilities, M. Landry for excellent graphical support, A. Jimenez for laboratory assistance, and T. Tanaka and B. Godin for helpful suggestions. These studies were supported by the following grants: DoDW81XWH-04-2-0035 Project 16 (M.F., M.C., X.L., E.T., R.B.), NASA SA23-06-017 (M.F., M.C., X.L., R.B., E.T., J.T., A.L., B.K.P.), State of Texas, Emerging Technology Fund (M.F., X.L., R.B., K.P.), and the National Institutes of Health (NIH) NCI 1R21CA1222864-01 (M.F., M.C., F.R.). The authors would like to recognize the contributions and support from the Alliance for NanoHealth (ANH).
Author information
Authors and Affiliations
Contributions
E.T. and M.F. conceived and designed all the experiments. E.T. and K.P. performed the loading and release experiments. E.T. performed the fluorescence and confocal microscopy, the multiple loading and releasing experiments and the delivery experiments on HUVEC cells. R.B. made the chemical modifications to particles and measured the zeta potential values. X.L. and M.C. made the porous silicon particles, conducted the SEM imaging and the BET analysis. A.L. and B.K.P. made and characterized the PEG-FITC-SWNTs under the guidance of J.T.. P.D. developed the mathematical modeling and E.T., M.F. and F.R. discussed the interpretation of results. E.T. wrote the draft paper and all co-authors helped in the revision of the paper.
Corresponding author
Ethics declarations
Competing interests
Commercialization rights on intellectual property presented in this paper have been acquired by Leonardo Biosystems Inc., from the title holder, the University of Texas Health Science Center in Houston. M.F. is the founding scientist of Leonardo Biosystems, and hereby discloses a financial interest in the company.
Rights and permissions
About this article
Cite this article
Tasciotti, E., Liu, X., Bhavane, R. et al. Mesoporous silicon particles as a multistage delivery system for imaging and therapeutic applications. Nature Nanotech 3, 151–157 (2008). https://doi.org/10.1038/nnano.2008.34
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2008.34
This article is cited by
-
Nanozymes as Catalytic Marvels for Biomedical and Environmental Concerns: A Chemical Engineering Approach
Journal of Cluster Science (2024)
-
Nanoparticle-mediated cancer cell therapy: basic science to clinical applications
Cancer and Metastasis Reviews (2023)
-
Nanozymes: Versatile Platforms for Cancer Diagnosis and Therapy
Nano-Micro Letters (2022)
-
Cell membrane protein functionalization of nanoparticles as a new tumor‐targeting strategy
Clinical and Translational Medicine (2019)