Abstract
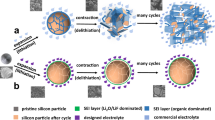
Although the performance of lithium ion-batteries continues to improve, their energy density and cycle life remain insufficient for applications in consumer electronics, transport and large-scale renewable energy storage1,2,3,4,5. Silicon has a large charge storage capacity and this makes it an attractive anode material, but pulverization during cycling and an unstable solid–electrolyte interphase has limited the cycle life of silicon anodes to hundreds of cycles6,7,8,9,10,11. Here, we show that anodes consisting of an active silicon nanotube surrounded by an ion-permeable silicon oxide shell can cycle over 6,000 times in half cells while retaining more than 85% of their initial capacity. The outer surface of the silicon nanotube is prevented from expansion by the oxide shell, and the expanding inner surface is not exposed to the electrolyte, resulting in a stable solid–electrolyte interphase. Batteries containing these double-walled silicon nanotube anodes exhibit charge capacities approximately eight times larger than conventional carbon anodes and charging rates of up to 20C (a rate of 1C corresponds to complete charge or discharge in one hour).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Armand, M. & Tarascon, J. M. Building better batteries. Nature 451, 652–657 (2008).
Goodenough, J. B. & Kim, Y. Challenges for rechargeable Li batteries. Chem. Mater. 22, 587–603 (2009).
Kang, B. & Ceder, G. Battery materials for ultrafast charging and discharging. Nature 458, 190–193 (2009).
Whittingham, M. S. Lithium batteries and cathode materials. Chem. Rev. 104, 4271–4301 (2004).
Tarascon, J. M. & Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 414, 359–367 (2001).
Beaulieu, L. Y., Eberman, K. W., Turner, R. L., Krause, L. J. & Dahn, J. R. Colossal reversible volume changes in lithium alloys. Electrochem. Solid State Lett. 4, A137–A140 (2001).
Besenhard, J. O., Yang, J. & Winter, M. Will advanced lithium-alloy anodes have a chance in lithium-ion batteries? J. Power Sources 68, 87–90 (1997).
Hatchard, T. D. & Dahn, J. R. In situ XRD and electrochemical study of the reaction of lithium with amorphous silicon. J. Electrochem. Soc. 151, A838–A842 (2004).
Raimann, P. R. et al. Monitoring dynamics of electrode reactions in Li-ion batteries by in situ ESEM. Ionics 12, 253–255 (2006).
Weydanz, W. J., Wohlfahrt-Mehrens, M. & Huggins, R. A. A room temperature study of the binary lithium–silicon and the ternary lithium–chromium–silicon system for use in rechargeable lithium batteries. J. Power Sources 81, 237–242 (1999).
Zhang, X. W. et al. Electrochemical performance of lithium ion battery, nano-silicon-based, disordered carbon composite anodes with different microstructures. J. Power Sources 125, 206–213 (2004).
Beaulieu, L. Y., Hatchard, T. D., Bonakdarpour, A., Fleischauer, M. D. & Dahn, J. R. Reaction of Li with alloy thin films studied by in situ AFM. J. Electrochem. Soc. 150, A1457–A1464 (2003).
Zhang, W. J. A review of the electrochemical performance of alloy anodes for lithium-ion batteries. J. Power Sources 196, 13–24 (2011).
Deshpande, R., Cheng, Y. T. & Verbrugge, M. W. Modeling diffusion-induced stress in nanowire electrode structures. J. Power Sources 195, 5081–5088 (2010).
Verbrugge, M. W. & Cheng, Y. T. Stress and strain-energy distributions within diffusion-controlled insertion-electrode particles subjected to periodic potential excitations. J. Electrochem. Soc. 156, A927–A937 (2009).
Cheng, Y. T. & Verbrugge, M. W. The influence of surface mechanics on diffusion induced stresses within spherical nanoparticles. J. Appl. Phy. 104, 083521 (2008).
Verbrugge, M. W., Cheng, Y. T. Stress distribution within spherical particles undergoing electrochemical insertion and extraction. Electrochem. Soc. Trans. 13, 127–139 (2008).
Chan, C. K. et al. High-performance lithium battery anodes using silicon nanowires. Nature Nanotech. 3, 31–35 (2008).
Cui, L. F., Ruffo, R., Chan, C. K., Peng, H. L. & Cui, Y. Crystalline–amorphous core–shell silicon nanowires for high capacity and high current battery electrodes. Nano Lett. 9, 491–495 (2009).
Cui, L. F., Yang, Y., Hsu, C. M. & Cui, Y. Carbon–silicon core–shell nanowires as high capacity electrode for lithium ion batteries. Nano Lett. 9, 3370–3374 (2009).
Hertzberg, B., Alexeev, A. & Yushin, G. Deformations in Si–Li anodes upon electrochemical alloying in nano-confined space. J. Am. Chem. Soc. 132, 8548–8549 (2010).
Kim, H., Han, B., Choo, J. & Cho, J. Three-dimensional porous silicon particles for use in high-performance lithium secondary batteries. Angew. Chem. Int. Ed. 47, 10151–10154 (2008).
Kim, H., Seo, M., Park, M. H. & Cho, J. A Critical size of silicon nano-anodes for lithium rechargeable batteries. Angew. Chem. Int. Ed. 49, 2146–2149 (2008).
Magasinski, A. et al. High-performance lithium-ion anodes using a hierarchical bottom-up approach. Nature Mater. 9, 353–358 (2010).
Park, M. H. et al. Silicon nanotube battery anodes. Nano Lett. 9, 3844–3847 (2009).
Song, T. et al. Arrays of sealed silicon nanotubes as anodes for lithium ion batteries. Nano Lett. 10, 1710–1716 (2010).
Aurbach, D. Review of selected electrode–solution interactions which determine the performance of Li and Li ion batteries. J. Power Sources 89, 206–218 (2000).
Verma, P., Maire, P. & Novak, P. A review of the features and analyses of the solid electrolyte interphase in Li-ion batteries. Electrochem. Acta 55, 6332–6341 (2010).
Choi, J. W. et al. Stepwise nanopore evolution in one-dimensional nanostructures. Nano Lett. 10, 1409–1413 (2010).
Chan, C. K., Ruffo, R., Hong, S. S. & Cui, Y. Surface chemistry and morphology of the solid electrolyte interphase on silicon nanowire lithium-ion battery anodes. J. Power Sources 189, 1132–1140 (2009).
Ruffo, R., Hong, S. S., Chan, C. K., Huggins, R. A. & Cui, Y. Impedance analysis of silicon nanowire lithium ion battery anodes. J. Phys. Chem. C 113, 11390–11398 (2009).
Szczech, J. R. & Jin, S. Nanostructured silicon for high capacity lithium battery anodes. Energy Environ. Sci. 4, 56–72 (2011).
Greiner, A. & Wendorff, J. H. Electrospinning: a fascinating method for the preparation of ultrathin fibres. Angew. Chem. Int. Ed. 46, 5670–5703 (2007).
Li, D. & Xia, Y. N. Electrospinning of nanofibers: reinventing the wheel? Adv. Mater. 16, 1151–1170 (2004).
Acknowledgements
This work was partially supported by the Assistant Secretary for Energy Efficiency and Renewable Energy, Office of Vehicle Technologies of the US Department of Energy (contract no. DE-AC02-05CH11231), and the Batteries for Advanced Transportation Technologies (BATT) Program (subcontract no. 6951379). This work is also partially supported by the SLAC National Accelerator Laboratory LDRD project. Y.C. acknowledges support from the King Abdullah University of Science and Technology (KAUST) Investigator Award (no. KUS-l1-001-12). G.C. acknowledges support from the Agency of Science, Technology and Research Singapore (A*STAR) National Science Scholarship. M.T.M. acknowledges support from the Stanford Graduate Fellowship, the National Science Foundation Graduate Fellowship and the National Defense Science and Engineering Graduate Fellowship.
Author information
Authors and Affiliations
Contributions
H.W. and Y.C. conceived the idea. H.W., G.C. and Y.Y. carried out materials fabrication and electrochemical tests. J.W.C. and M.T.M. performed TEM measurements. I.R. and H.W. designed and carried out the simulations and analysed data. A.J performed Auger measurements. H.W. and Y.C. co-wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 4804 kb)
Supplementary information
Supplementary movie 1 (AVI 842 kb)
Supplementary information
Supplementary movie 2 (AVI 4047 kb)
Rights and permissions
About this article
Cite this article
Wu, H., Chan, G., Choi, J. et al. Stable cycling of double-walled silicon nanotube battery anodes through solid–electrolyte interphase control. Nature Nanotech 7, 310–315 (2012). https://doi.org/10.1038/nnano.2012.35
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2012.35
This article is cited by
-
Si-Based Anodes: Advances and Challenges in Li-Ion Batteries for Enhanced Stability
Electrochemical Energy Reviews (2024)
-
The effects of B-doping on the structure and performance of Si composite anode material
Journal of Materials Science: Materials in Electronics (2024)
-
Effect of Si Doping and Active Carbon Surface Modifications on the Structure and Electrical Performance of Li4Ti5O12 Anode Material for Lithium-Ion Batteries
Ionics (2024)
-
Surface-redox sodium-ion storage in anatase titanium oxide
Nature Communications (2023)
-
Nanoporous silicon fiber networks in a composite anode for all-solid-state batteries with superior cycling performance
Scientific Reports (2023)